A Medical Device Quality Management System (QMS) is a set of policies, processes, and procedures that a manufacturer of medical devices has in place to ensure that their products are safe and effective for their intended use.
The QMS includes all aspects of design and development, manufacturing, supplier management, risk management, complaint handling, clinical data, storage, distribution, product labeling, and other areas.
Medical devices have different classes based on their risk to the end user. Specific regulatory requirements are applicable depending on the device classification to ensure safe and reliable products.
A medical device QMS needs to reflect the applicable requirements of a company’s processes to help ensure regulatory compliance.
This article will discuss a medical device quality management system, its purpose, some of the main regulatory requirements, and the role of an eQMS in helping streamline processes and maintain a compliant medical device quality system.
SimplerQMS provides medical device QMS software. Our solution includes all QMS modules into one system to streamline processes, including document control, change control, employee training, audits, nonconformance, CAPA management, and much more.
If you are interested in discovering how your company can benefit from SimplerQMS, book a personalized demo and talk with our software experts.
In this article, we will be looking at the following:
- What Is a Medical Device Quality Management System (QMS)?
- Purpose of Medical Device Quality Management System
- International Standards and Regulations Governing Medical Device QMS
- Major Medical Device QMS Processes
- Role of Medical Device Quality Management Software
- Frequently Asked Questions (FAQs) About Medical Device QMS
What Is a Medical Device Quality Management System (QMS)?
A Medical Device Quality Management System (QMS) is a structured system that documents the procedures and processes implemented throughout the lifecycle of a medical device.
A medical device QMS is essential to ensure quality in developing, manufacturing, and distributing processes.
As a manufacturer of medical devices planning to sell your products in the international market, you are required to implement and maintain a quality management system compliant with applicable international and national standards, guidelines, and regulations.
This requires establishing quality processes and assigning responsibilities, resources, and timeframes for each process.
Some of the main QMS processes for medical device manufacturers include document control, change control, employee training, audit management, CAPA, supplier management, risk management, and complaint handling.
Quality Management System (QMS) can be implemented using paper documents or eQMS software.
Medical device manufacturers can manually manage their QMS if they have the resources to handle manual paperwork.
But with the increasing number and complexity of regulatory requirements, managing all quality processes relying on manual paperwork can become challenging.
Many companies are migrating to eQMS and taking advantage of the benefits, including improved workflows, reduced time and costs, and cloud-based document storage.
SimplerQMS offers an eQMS solution designed for the medical device industry to help companies streamline their quality processes and achieve compliance. We provide interconnected QMS modules for more streamlined control and management of documents, changes, employee training, nonconformances, CAPAs, suppliers, and many other processes.
Purpose of Medical Device Quality Management System
The purpose of a medical device quality management system is to ensure that products are safe and effective for their intended use.
A QMS provides a framework for consistent and documented processes throughout the product lifecycle, from design and development to post-market surveillance.
Implementing and maintaining a QMS for medical devices is critical for manufacturers seeking approval from regulatory authorities in their intended market.
In Europe, having a QMS is required to obtain the CE marking and be able to sell your device across the European Economic Area. It is necessary to achieve compliance with the applicable EU regulations, such as the Medical Device Regulation (MDR) or In Vitro Diagnostic Regulation (IVDR).
You can learn more about how to obtain CE marking for medical devices in our step-by-step guide.
The Food and Drug Administration (FDA) has set forth current good manufacturing practice (cGMP) requirements for medical device quality management systems in the United States.
These requirements are outlined in the 21 CFR Part 820, also known as Quality System Regulation (QSR). They must be implemented throughout the product lifecycle.
International Standards and Regulations Governing Medical Device QMS
There are numerous international standards and regulations governing medical device quality management systems.
This article will focus on two major international markets, Europe and the United States.
Medical device manufacturers marketing and selling in the EU will most likely comply with ISO 13485:2016 standard, MDR, and IVDR for requirements for quality management systems.
On the other hand, manufacturers selling in the US will comply with the FDA’s regulation 21 CFR Part 820, also known as Quality System Regulations (QSR).
Let’s take a brief look at the major standards and regulations that you must be aware of:
- ISO 13485:2016 Standard
- Medical Device Regulation (MDR)
- In Vitro Diagnostic Regulation (IVDR)
- FDA 21 CFR Part 820 Regulation
ISO 13485:2016 Standard
The International Organization for Standardization (ISO) is responsible for the international regulatory standard ISO 13485:2016. It specifies the requirements for a QMS that can be used by organizations involved in the life cycle of a medical device.
Manufacturers that comply with this standard demonstrate their ability to provide medical devices and related services that constantly meet customer and applicable regulatory requirements.
You can read our article to learn more about an ISO 13485:2016 compliant QMS. It discusses the purpose of the ISO 13485:2016 standard, the main QMS requirements, and the role of eQMS in supporting compliance.
And if you want to know specifically about audits, check out our ISO 13485:2016 audits article for an overview of different types of audits and the execution process.
Medical Device Regulation (MDR)
The Medical Devices Regulation (MDR), or EU 2017/745, specifies the requirements for designing, manufacturing and distributing medical devices and accessories for human use in the EU.
The MDR requires medical device manufacturers to establish and maintain a comprehensive QMS to ensure compliance.
Although not mandatory in the MDR, manufacturers often use compliance with the ISO 13485:2016 standard since it presumes compliance with QMS requirements presented in the regulation. Which can help speed up the MDR certification process.
You can learn more about this regulation by reading our article on EU MDR Quality Management System.
In Vitro Medical Device Regulation (IVDR)
The In Vitro Diagnostic Medical Devices Regulation (IVDR) is the most common way to refer to Regulation (EU) 2017/746.
The IVDR has more stringent requirements for manufacturers of in vitro diagnostic devices, including a focus on risk-based classification, performance evaluation, and post-market surveillance.
It also requires manufacturers of in vitro diagnostic devices to implement a QMS that ensures compliance with the essential principles of safety and performance.
Like the MDR, the IVDR also recognizes compliance with ISO 13485:2016 for QMS requirements.
Feel free to read our article to learn more about EU IVDR in a detailed introduction.
FDA 21 CFR Part 820 Regulation
The 21 CFR Part 820 is a regulation of the US Food and Drug Administration (FDA) that outlines the Quality System Regulation (QSR) requirements for medical device manufacturers.
This regulation specifies the minimum requirements for a medical device QMS to ensure that devices are safe and effective for their intended use.
Compliance with 21 CFR Part 820 is mandatory for all medical device manufacturers who wish to sell their products in the US market.
Our article on the FDA 21 CFR Part 820 Quality System Regulation – provides more information regarding this regulation and the role of eQMS in supporting compliance.
Additionally, other requirements must be considered when implementing a quality management system using a computer system, such as QMS software or an eQMS.
This includes ensuring the software is validated and all necessary security measures are in place to prevent unauthorized access and data tampering, among other things.
The software should also be periodically reviewed and updated to ensure it remains compliant with regulatory requirements.
Relevant requirements concerning a QMS system validation are the following:
- FDA 21 CFR Part 11
- EU GMP Annex 11
- ISPE GAMP5
- ISO 13485:2016 Clause 4.1.6
- FDA 21 CFR Part 820.70(i)
FDA 21 CFR Part 11
The 21 CFR Part 11 regulates how electronic records are created, modified, maintained, and stored.
It specifies the requirements to ensure the accuracy and security of electronic records, including the validation of open and closed systems.
This regulation also sets the guidelines for electronic signatures, which are legally binding and serve as an equivalent to traditional handwritten signatures.
You can read our article on the 21 CFR Part 11 requirements to learn more about this regulation.
EU GMP Annex 11
The EU GMP Annex 11 provides Good Manufacturing Practices (GMP) guidelines for computerized systems.
It specifies the requirements to ensure computer systems do not cause a reduction in product quality, process control, or quality assurance, nor increase the overall risk of the process.
The validation documentation must contain change control records and reports on any deviations detected during the validation process.
Having an updated inventory of all relevant systems and their GMP functionality is also important.
ISPE GAMP5
The Good Automated Manufacturing Practice 5 (GAMP5) is a guideline developed by the International Society for Pharmaceutical Engineering (ISPE) for computerized systems.
It provides a risk-based approach to the validation and maintenance of computerized systems to ensure their accuracy, reliability, and consistency.
SimplerQMS software is fully validated according to ISPE GAMP5. It is re-validated upon creating a new version or applying standard updates. This helps you to save time to focus on more value-added activities.
Our solution also complies with FDA 21 CFR Part 11 and EU GMP Annex 11 for electronic records, electronic signatures, and computerized system manufacturing guidelines.
ISO 13485:2016 Clause 4.1.6
Clause 4.1.6 in the ISO 13485:2016 establishes that the medical device manufacturer must document procedures for validating computer software used in the QMS before initial use and after any changes to the software.
The specific approach and activities for software validation and revalidation should correspond to the risk associated with its use.
FDA 21 CFR Part 820.70(i)
The 21 CFR Part 820 section 70(i) also specifies that manufacturers must validate computer software for its intended use according to an established protocol.
All software changes must be validated before approval and issuance, documenting all validation activities and results.
Major Medical Device QMS Processes
We will focus only on some of the major medical device QMS processes in accordance with international standards and regulations – the ISO 13485:2016 standard and the FDA 21 CFR Part 820 regulation.
Among several QMS processes, some of them are mentioned in both ISO 13485:2016 and FDA 21 CFR Part 820. This section will briefly discuss only some of these quality processes and their requirements.
NOTE
Please note that many more standards, guidelines, and regulations may apply to your business. Always refer to the applicable requirements for official information.
According to these, the major medical device quality management requirements can be summarized as given in the following subsections:
- Document Control
- Training Management
- Audit Management
- CAPA Management
Document Control
Your medical device quality management system should include processes and procedures for managing the many different documents that move within your company, amongst sponsors, investors, and regulatory agencies throughout the lifecycle of your product.
This entire process is referred to as document control.
Over the past decade, many manufacturers have migrated from traditional paper-based QMS to automated medical device quality management software with robust document control capabilities.
Such software automatically collects data, routes documents for review and approval processes of various documents, and stores them in a single storage location.
QMS software, such as SimplerQMS, provides automatic version control, which ensures that only the latest version of the documents is in circulation.
SimplerQMS offers pre-configured workflows to simplify document control. You can easily create documents using forms or document templates and route them for review as often as needed. After the final version of a document is approved and released, employees are automatically notified.
Moreover, if you want to know more about document control, read our articles on Quality Management System Documentation and Medical Device Document Control.
Training Management
International regulations and standards such as the FDA 21 CFR 820 and ISO 13485:2016 require medical device manufacturers to have specifically trained and competent employees doing their job.
This means that all training procedures must be constantly evaluated, and all employee training records must be appropriately maintained.
For instance, changes to the manufacturing process of medical devices must be learned by all personnel involved. This means companies must provide training and assess the training effectiveness to ensure employees have the skill to perform their duties.
SimplerQMS provides training management software to help streamline the entire training process. From assigning training tasks to specific employees, distributing training material, and tracking all training activities, to providing quizzes to measure training effectiveness.
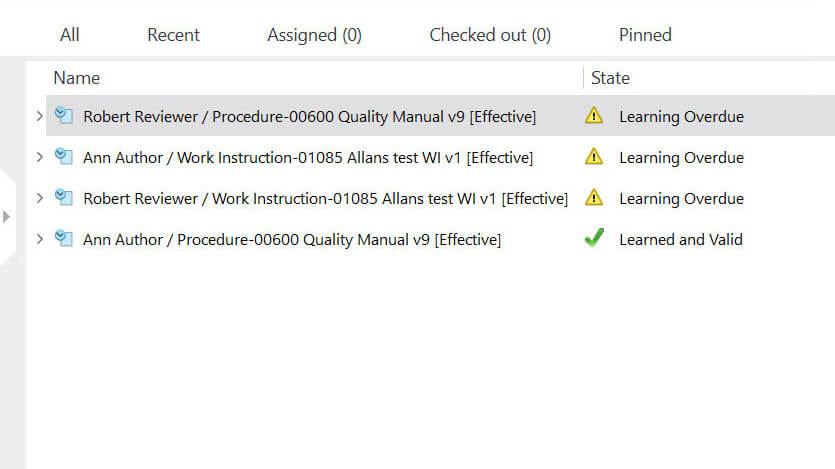
Audit Management
The FDA 21 CFR 820 and ISO 13485:2016 require medical device manufacturers to establish a proper audit process and conduct regular quality audits.
This is to ensure that the medical device quality management system as a whole is compliant.
Companies need to properly audit all the applicable requirements for a medical device to ensure compliance regarding product safety and efficacy.
The audit management module by SimplerQMS facilitates the entire audit process. You can schedule activities, set due dates, send automatic notifications to the right personnel, and track employee performance related to their assignments.
Whenever an audit finding occurs, you will be able to easily link it to a nonconformance case, supplier corrective action request (SCAR), or escalate to corrective action and preventive action (CAPA).
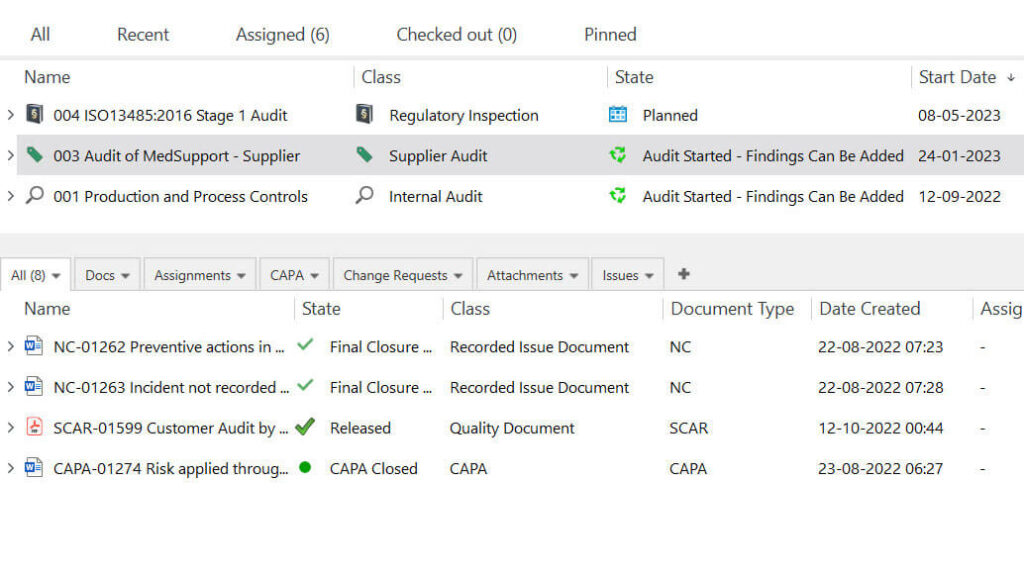
You can read our article on medical device audits to learn more about the different audits in the medical device industry.
CAPA Management
As per the FDA 21 CFR 820 and the ISO 13485:2016 requirements, all manufacturers of medical devices must implement the process of managing corrective actions and preventive actions (CAPA).
The CAPA process is essential to resolve and prevent issues that are likely to impact the quality of the product, such as nonconformances, audit findings, complaints, etc. Additionally, all actions must be documented adequately and resolved in a timely manner.
Key CAPA process steps are illustrated below.
With the SimplerQMS CAPA management software, you can streamline the entire CAPA process by automating data collection, routing, follow-ups, notifications, approvals, and escalation of overdue activities.
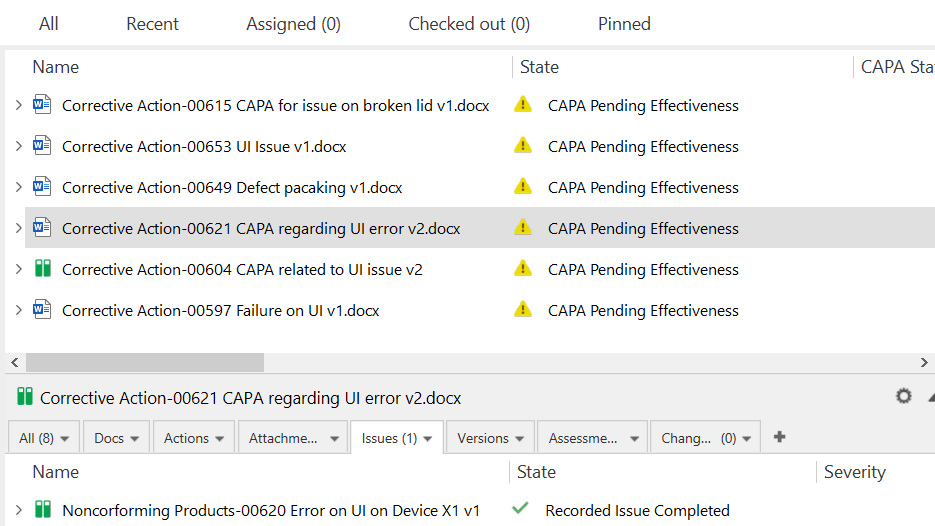
You can quickly initiate CAPA forms directly from issues, such as nonconformances or deviations, audit findings, and customer complaints.
The system automatically enters data from the metadata in the CAPA form to reduce human error. It also enables you to relate documents in scope and eliminates the need to circulate documents or chase signatures manually.
Check out our article to better understand what CAPA is in the medical device industry.
In addition to the quality processes mentioned in this section, several other processes are required as per standards and regulations, such as ISO 13485:2016 and FDA 21 CFR Part 820.
Some of these include:
- Change management
- Complaint management
- Nonconformance management
- Equipment management
- Supplier management
This article does not cover all sections above. Companies should always verify which processes are relevant to them and comply with the specific requirements that apply to their business.
Role of Medical Device Quality Management Software
A medical device quality management system is essential for ensuring products are safe and effective for their intended use.
It is a requirement of many standards and regulations that medical device companies have a compliant QMS.
Companies can have a paper-based or hybrid QMS by implementing a manual system. The latter approach may be adequate for small companies with adequate resources, but it can be time-consuming, prone to errors, and challenging to maintain compliance with regulatory requirements.
Electronic QMS provides an alternative solution that offers greater efficiency and accuracy, making it a robust option for numerous companies.
Medical device manufacturers can use such software to manage quality processes throughout the product lifecycle, from design and development to manufacturing, labeling, distribution, and corrective and preventive actions.
The benefits of medical device quality system software include the following:
- Ensure compliance with regulatory requirements using pre-configured workflows
- Improve customer satisfaction by addressing issues in a timely manner
- Save time with automated documentation processes
- Ensure traceability with automated data collection and flexible linking capabilities
- Improve communication and collaboration across departments
- Reduce the risk of inaccuracies by mitigating human errors associated with manual data entry
SimplerQMS provides an eQMS designed for the medical device industry. Our software streamlines quality processes and helps companies in achieving compliance with regulatory requirements.
We offer robust document management capabilities that simplify document creation, versioning, review, approval, and search within one secure location.
Our QMS modules are interconnected and allow for easy linking of complaints, CAPAs, training, and other quality management processes.
The SimplerQMS system is fully validated in compliance with ISPE GAMP5 and undergoes revalidation for each new version or standard update.
It also complies with FDA 21 CFR Part 11, which sets the guidelines for electronic signatures and electronic records, and EU GMP Annex 11, which provides good manufacturing guidelines for computerized systems.
Now that you are aware of the benefits of an eQMS in terms of compliance and streamlined workflows, it is important to consider the associated time and cost savings.
Understanding these factors will help you make an informed decision about whether to allocate a budget for an eQMS and move forward with implementation.
We recommend downloading our eQMS Business Case template. This tool will help you provide decision-makers with concrete evidence of the benefits of implementing an eQMS and make a compelling case for the investment.
Frequently Asked Questions (FAQs) About Medical Device QMS
What Are the Two Main Quality Systems Adopted by Medical Device Manufacturers?
The two main quality systems medical device manufacturers adopt are the FDA 21 CFR 820 and the ISO 13485:2016.
The FDA 21 CFR 820 sets the requirements for quality management systems in the US, while ISO 13485:2016 is an international regulatory standard that outlines the requirements for QMS for medical devices in the EU and other countries.
What is the Best Quality Management Software for Medical Devices?
The best medical device QMS software for your company will depend on various factors, such as the size and complexity of your company, applicable specific requirements, available time, and budget.
QMS software solutions are available for Life Science companies in the medical device, pharmaceutical, biotechnological, and other industries. Each solution has unique features and qualities, so it is essential to consider your company’s specific needs and regulatory requirements.
The chosen software should be easy to use and provide good customer support to help with any doubts or problems that may arise. For a more straightforward evaluation, there is a compiled list of the 6 best QMS software solutions for Life Sciences options available.
Additionally, our QMS software comparison template can be used to compare shortlisted options and select the best one for your company or as a reference to develop a list of requirements for quality management software.
What Is the ISO 13485:2016 Quality Management System?
The ISO 13485:2016 QMS standard specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that constantly meet customer and applicable regulatory requirements.
You can learn more about this international regulatory standard in our article on ISO 13485:2016 Compliant QMS.
When Is a Quality Management System (QMS) Required for Medical Devices?
Medical device companies require a Quality Management System (QMS) when registering devices for marketing and selling.
Compliance with FDA 21 CFR Part 820 is required for operating in the US market, and medium to high-risk devices must go through the 510(K) process.
In Europe, obtaining the CE marking is required for marketing and selling devices, and certification to ISO 13485:2016 can be used to comply with the QMS requirements of the MDR or IVDR.
When Should I Start Building a Medical Device QMS?
Timing is crucial when building a QMS.
Starting the QMS during the early prototype phase or idea testing can put unnecessary constraints on development. Sufficient research and knowledge should be obtained before building the QMS.
However, it is also not advisable to wait too long to build the QMS, as it takes time to develop a suitable system.
The ideal time to start building a medical device QMS is when enough information and knowledge have been gathered to ensure an efficient QMS development process. This can be around the Design and Development phase.
How Long Does It Take to Fully Implement Medical Device QMS?
It typically takes 3 to 9 months to fully establish a medical device QMS. Still, medical device consultants suggest starting preparations at least 18-24 months before commercialization.
Using a document template for processes and protocols is advantageous and can help speed up the implementation.
SimplerQMS offers a customizable document template package based on Life Science requirements, such as ISO 13485:2016, to simplify the creation of documents and streamline your quality documentation processes by managing forms and templates effectively.
Final Thoughts
The medical device quality management system is a regulatory requirement for manufacturers of medical devices. The quality management system covers all aspects of the medical device’s lifecycle.
It is a regulated framework comprising strategies and procedures for the design, clinical data, manufacturing, storage and distribution, supplier management, complaints, labeling, and other aspects of your medical devices.
SimplerQMS medical device QMS software streamlines your quality management processes by managing routine documentation tasks, enabling you to achieve greater efficiency while making compliance easier.
If you are looking for a comprehensive QMS software solution to manage quality processes more effectively, schedule a demo presentation to see SimplerQMS in action and talk to our experts.