Quality Management Software for
life sciences
The QMS Software Built by Life Science Experts
SimplerQMS is the QMS software for life science companies – helping you boost productivity by up to 50% and reduce costs.
Book a Demo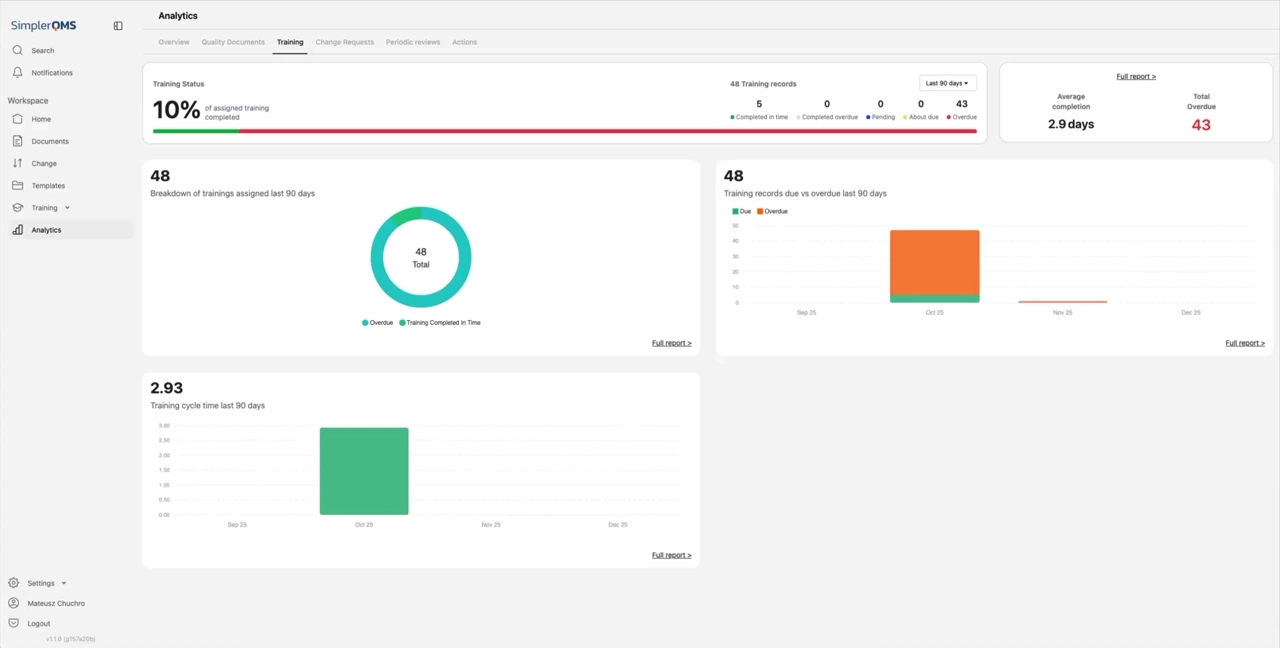
Take a 3-minute tour and see SimplerQMS in action.
Trusted by 5,000+ life science professionals worldwide – and growing.
15,000+ life science professional community
4.6 rating on Capterra
4.9 rating on G2
Case study
Case study
Case study
Case study
Case study
QMS Software Built for Life Science Industries
SimplerQMS is designed for regulated life science sectors, including pharma, biotech, medical devices, laboratories, and CROs/CMOs.
Pharmaceutical and Biotechnology
Manage GMP-compliant documentation, batch records, training, and CAPA in one validated quality management software. SimplerQMS supports compliance with FDA 21 CFR Parts 210–211, ICH Q10, and related GMP requirements.
Explore QMS Software for Pharma & Biotech
Medical Device
Manage design control, risk management, and post-market activities under one integrated QMS software. SimplerQMS supports compliance with ISO 13485, FDA 21 CFR Part 820, EU MDR/IVD, and other requirements.
Explore QMS Software for Medical Devices
Clinical and Medical Laboratories
Manage document control, equipment records, training, and other quality processes in one QMS software. SimplerQMS supports compliance with ISO 15189, CLIA and related requirements applicable to clinical and medical laboratories.
Explore QMS Software for Laboratories
Contract Research, Development, and Manufacturing
Manage quality documentation, audits, protocols, and other quality processes in one centralized quality management solution. SimplerQMS supports compliance with GxP requirements and sponsor expectations across outsourced research and manufacturing activities.
Explore QMS Software for CROs and CDMOs
Guiding Life Science Companies Toward Compliance and Quality Excellence
SimplerQMS helps life science companies simplify compliance and strengthen quality through a fully validated QMS software and expert, hands-on support.
QMS Software Supporting FDA, ISO, and GxP Compliance
SimplerQMS helps life science companies ensure compliance with FDA, ISO, EU, GxP, and other regulatory requirements through a fully validated QMS software.
ISO 13485:2016
SimplerQMS A/S is certified to ISO 13485:2016, and the SimplerQMS software is designed to support and align with the requirements of the ISO 13485 quality management system.
ISO/IEC 27001:2022
SimplerQMS A/S is certified to ISO/IEC 27001:2022 and implements information security controls aligned with the standard across its organization and QMS software platform.
FDA 21 CFR Part 11
SimplerQMS complies with FDA 21 CFR Part 11.
FDA 21 CFR Part 820
SimplerQMS supports compliance with FDA 21 CFR Part 820.
FDA 21 CFR Part 211
SimplerQMS supports GMP requirements under FDA 21 CFR Part 211.
FDA 21 CFR Part 212
SimplerQMS supports compliance with FDA 21 CFR Part 212.
FDA 21 CFR Part 4
SimplerQMS support compliance with FDA 21 CFR Part 4.
FDA Data Integrity
SimplerQMS supports FDA Data Integrity Guidance (FDA-2018-D-3984) principles.
CLIA
SimplerQMS supports adherence to CLIA.
EudraLex Volume 4 GMP
SimplerQMS supports compliance with EudraLex Volume 4 GMP (Part 1 – Medicinal Products).
EudraLex GMP Annex 11
SimplerQMS supports compliance with EudraLex GMP Annex 11: Computerized Systems, Annex 16 QP certification for batch release, and Annex 19 reference/retention samples.
EU MDR
SimplerQMS supports compliance with EU MDR.
EU IVDR
SimplerQMS supports compliance with EU IVDR.
UK MDR
SimplerQMS empowers life science organizations to meet UK MDR requirements.
ISO 14971:2019
SimplerQMS facilitates adherence to ISO 14971:2019.
IEC 60601
SimplerQMS supports compliance with IEC 60601.
IEC 62304
SimplerQMS supports alignment with IEC 62304.
ISO 1518:2022
SimplerQMS provides functionality aligned with ISO 1518:2022.
ISO 16085:2021
SimplerQMS supports compliance with ISO 16085:2021.
ICH Q10
SimplerQMS aligns with pharmaceutical quality systems requirements per ICH Q10.
ICH Q9
SimplerQMS supports risk management per ICH Q9.
ICH Q8
SimplerQMS supports compliance per ICH Q8.
PIC/S GMP
SimplerQMS supports compliance with PIC/S GMP (Part 1 – Medicinal Products).
ISPE GAMP 5
SimplerQMS meets ISPE GAMP 5 guidelines, including Annex D1, Section 3 and Annex M3.
ISO 9001:2015
SimplerQMS supports compliance with ISO 9001:2015.
ISO 90003:2018
SimplerQMS applies ISO 90003:2018 best practices.
ISO/IEC 27002:2022
SimplerQMS follows ISO/IEC 27002:2022 guidance.
GDPR
SimplerQMS supports alignment with GDPR (EU).
HIPAA
SimplerQMS supports alignment with HIPAA (US).
SimplerQMS Transforms Your Quality Management into a Connected QMS
See the difference between manual or generic QMS software systems and a connected, fully validated quality management software built specifically for life science companies.
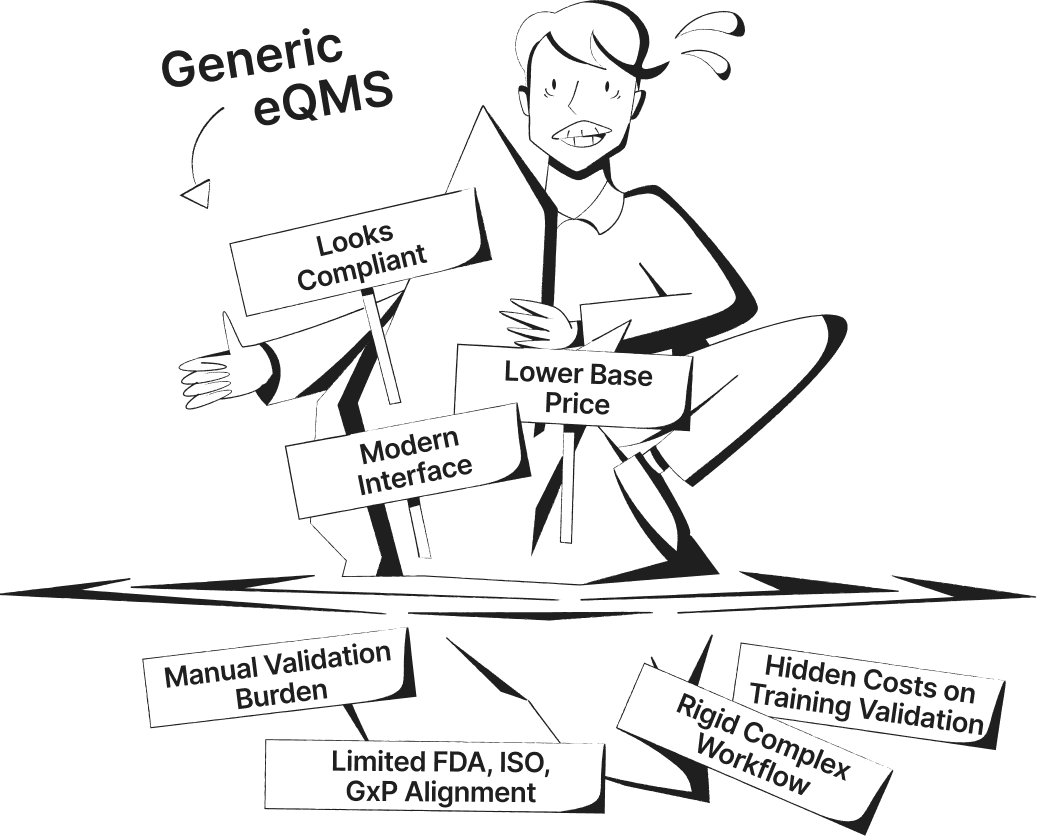
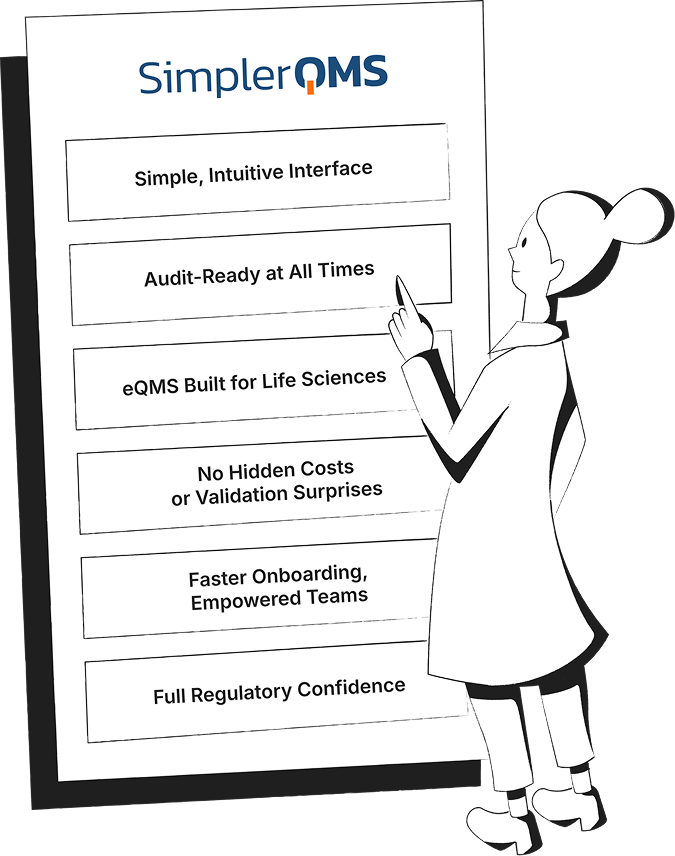
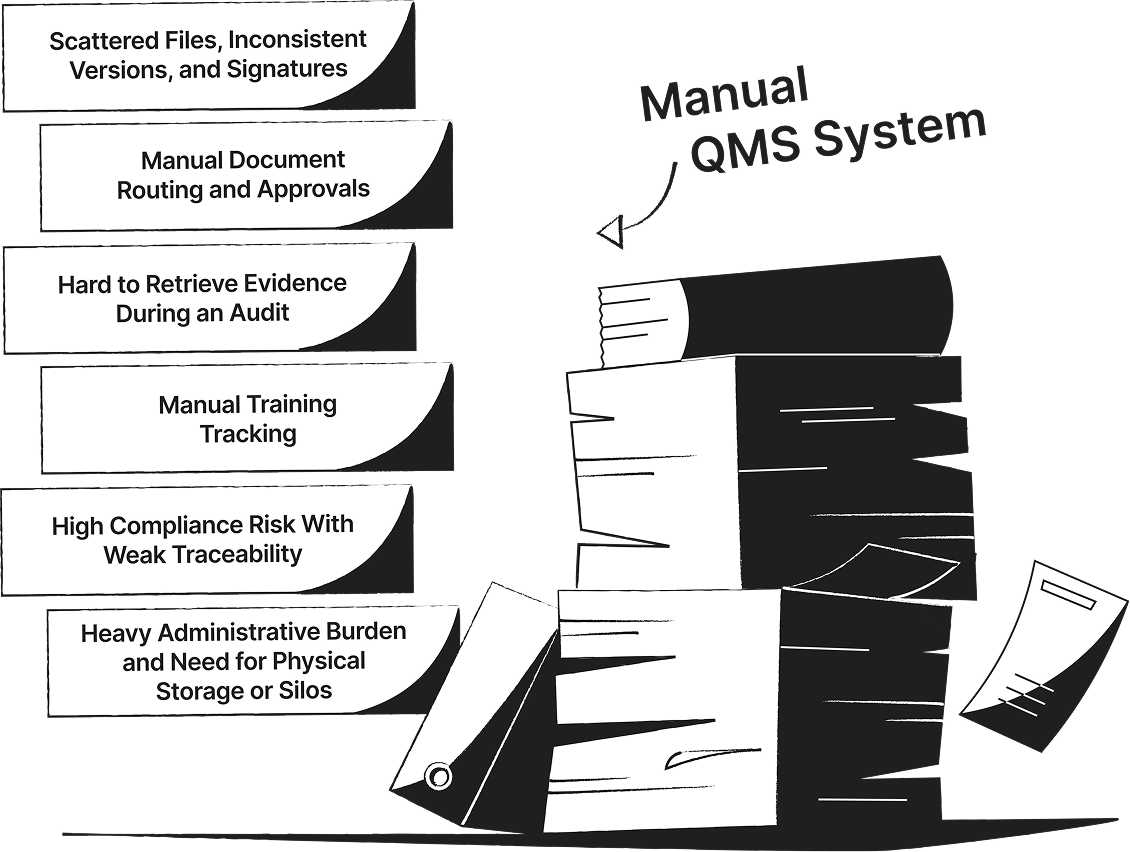

Streamline Quality Processes with QMS Software Modules in SimplerQMS
SimplerQMS QMS modules streamline quality processes while strengthening compliance and traceability.
Explore Core Features of Our Cloud-Based Quality Management Platform
Discover the core capabilities that help life science teams streamline documentation, workflows, integrations, and compliance in a secure cloud-based QMS.
Native Microsoft 365 and Copilot AI Integration
Integrate seamlessly with Microsoft 365 and Microsoft Copilot (AI Assistant) to create, review, and collaborate on documents directly in Word, Excel, and PowerPoint.

Electronic Signatures and Complete Audit Trails
Use built-in e-signatures and time-stamped audit logs compliant with FDA 21 CFR Part 11 and EU GMP Annex 11 to ensure data integrity and full traceability.
Learn about electronic signatures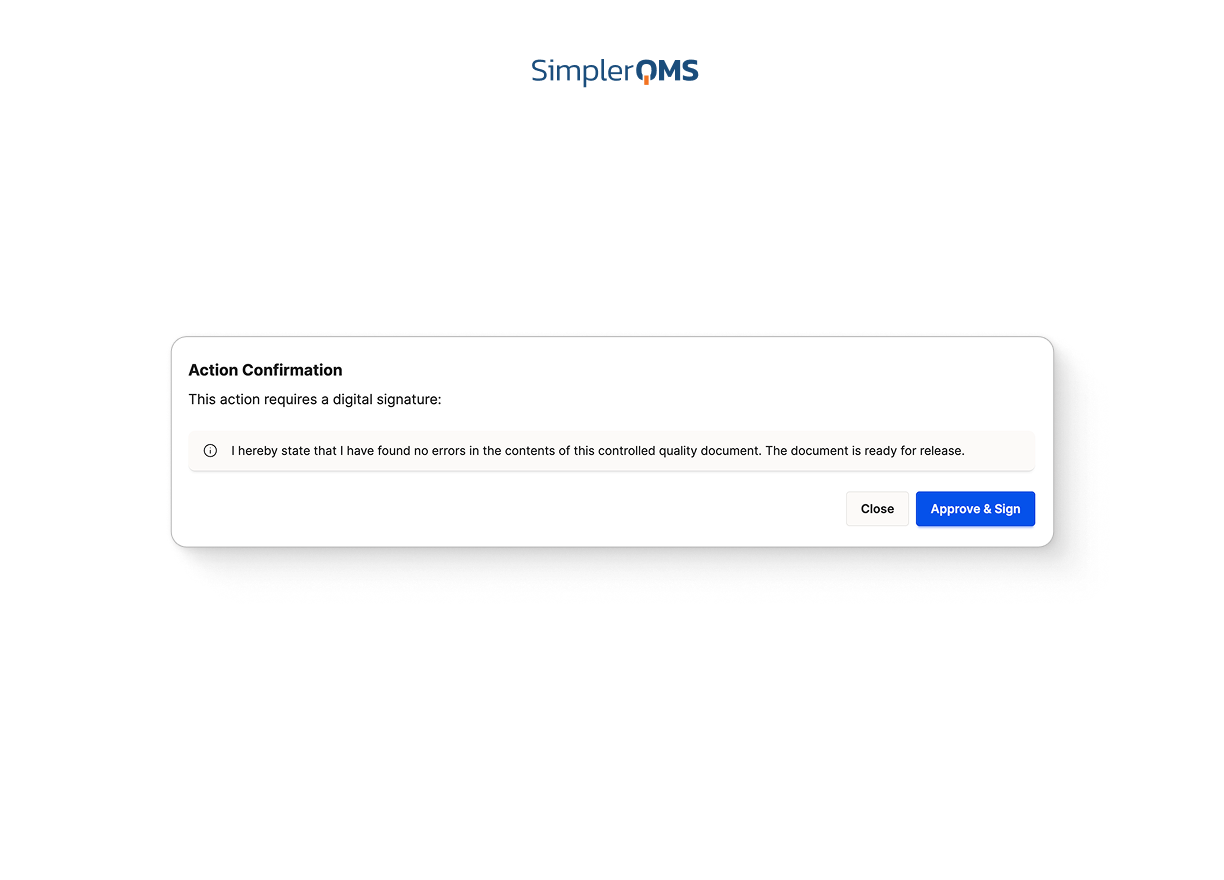
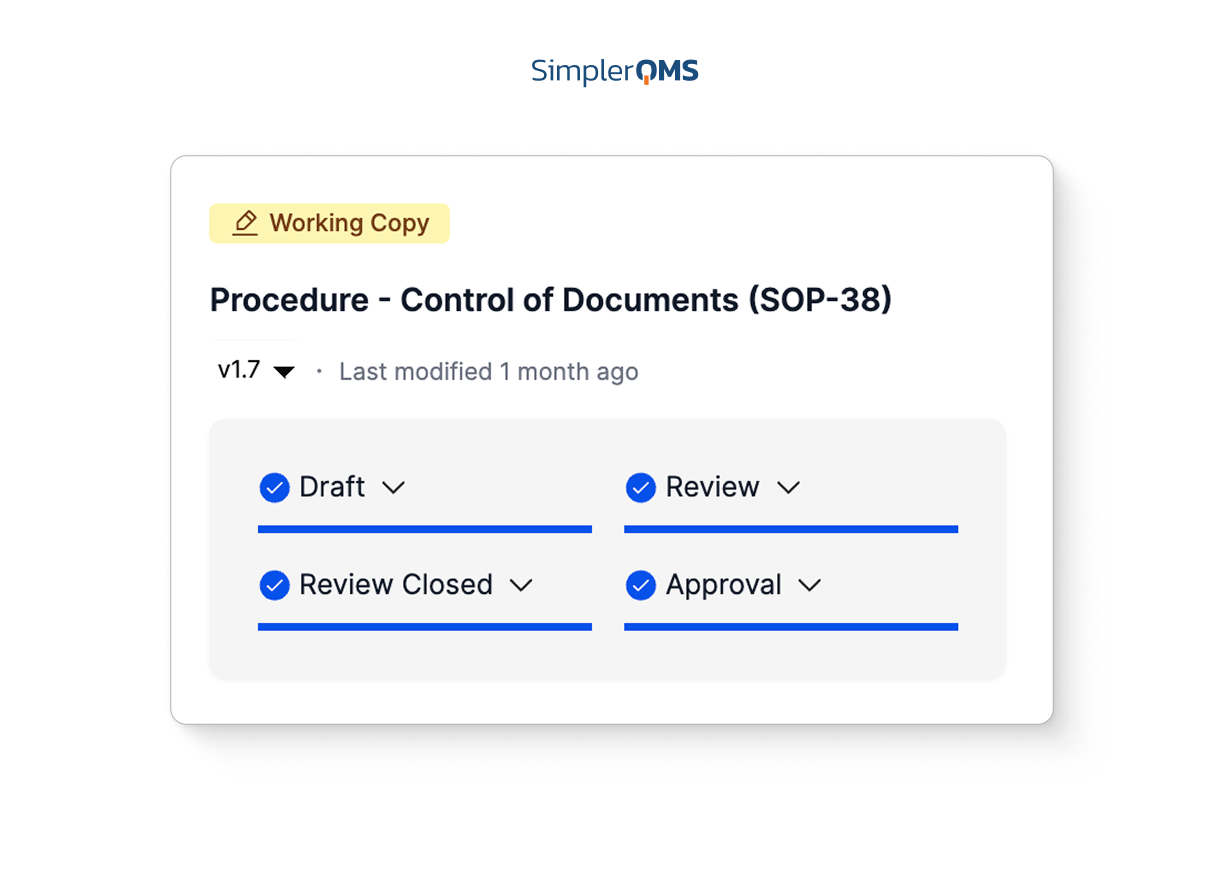
Automated Workflows and Linked Records
Automate reviews, approvals, and routing while linking related records to maintain traceability across all quality processes.
Centralized Dashboards and Reporting
Visualize key metrics and KPIs through real-time dashboards that drive data-based decisions and continuous improvement.
Learn about analytics and reporting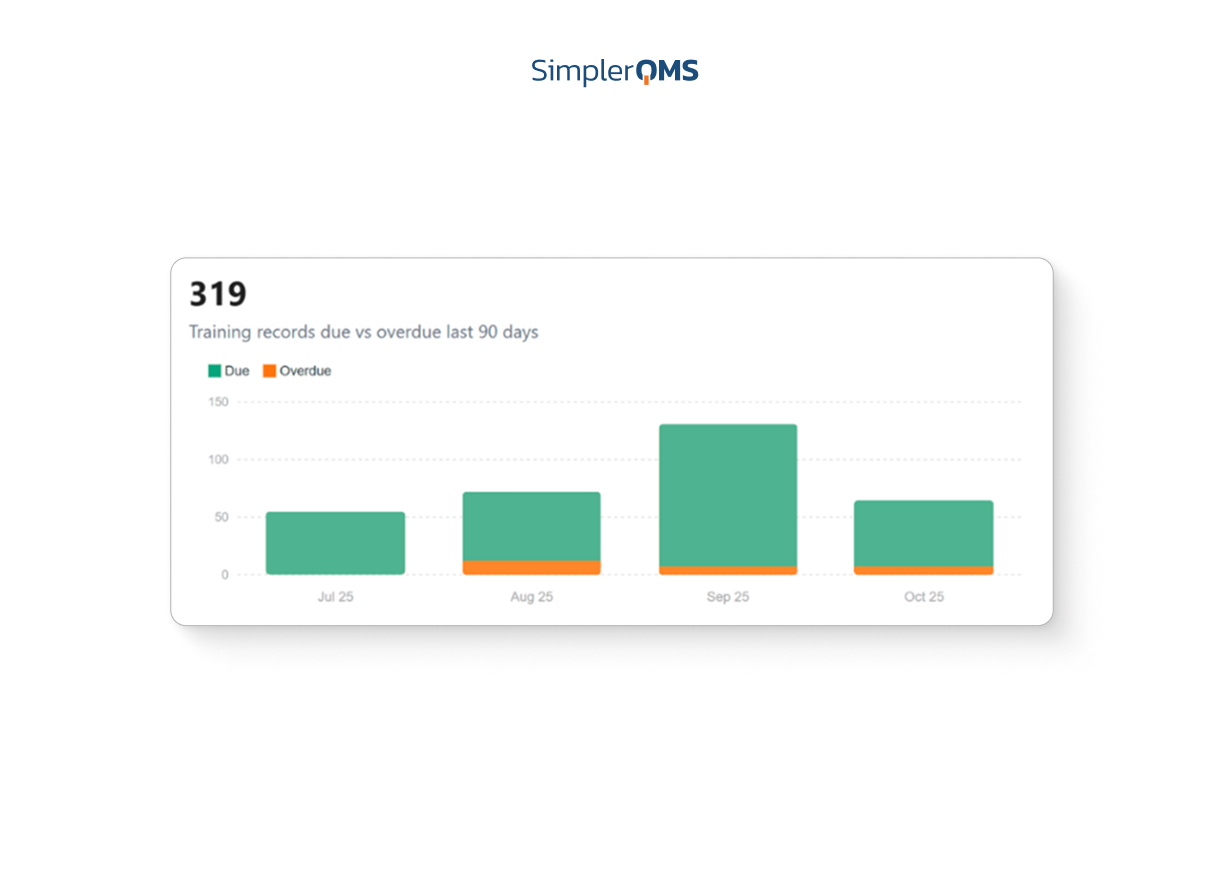
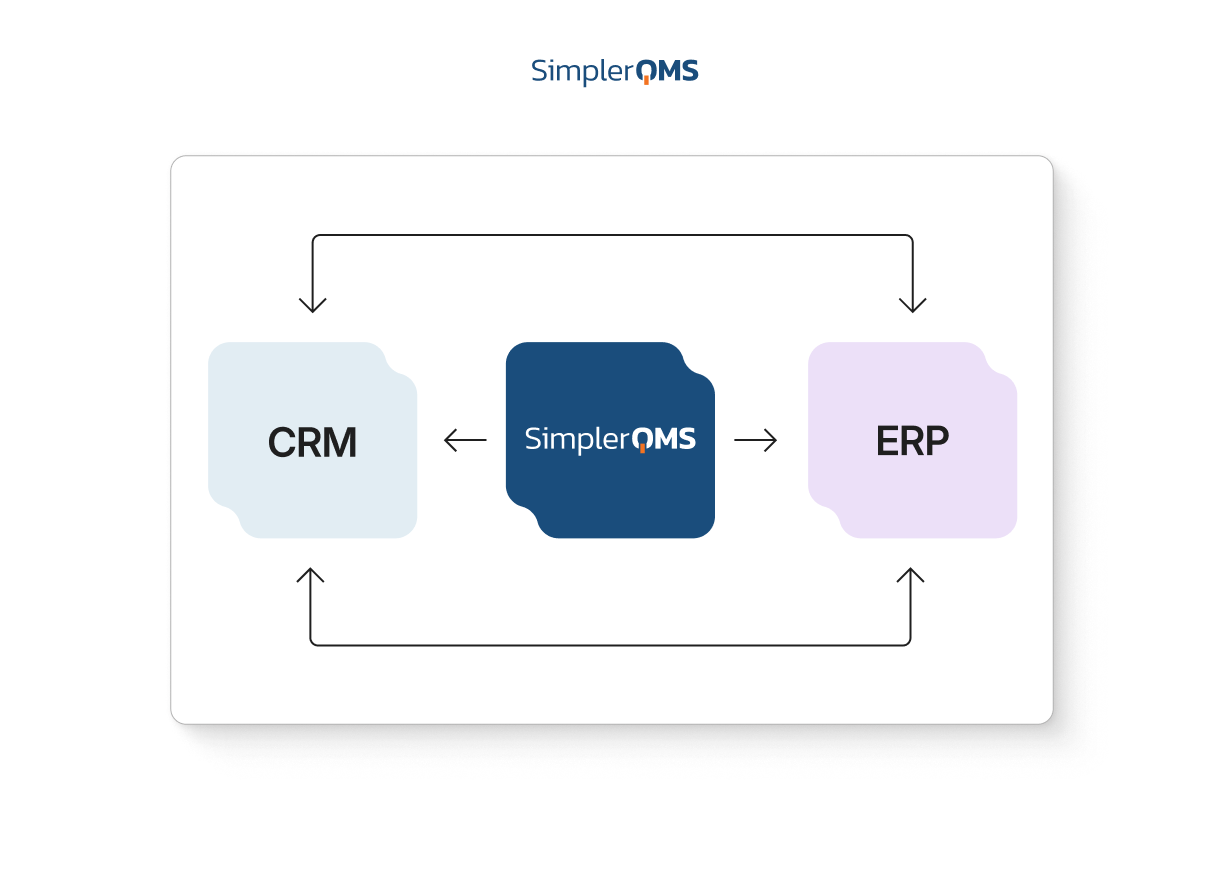
Integrations With Your Existing Systems
SimplerQMS supports integration with ERP, CRM, PLM, WMS, and other applications through an application programming interface (API).
Empower Every Team with One Connected Quality Management Solution
SimplerQMS unifies Quality, Regulatory, R&D, Manufacturing, and Management teams in one validated QMS software for life science companies.
Quality Assurance
Quality Assurance teams maintain control over documents, CAPA, deviations, audits, and training through structured workflows and real-time visibility across the QMS.
Key benefits are listed below.

Regulatory Affairs
Regulatory Affairs professionals manage document control, changes, and regulatory documentation with full traceability to support inspections and regulatory submissions.
Key benefits are listed below.

Research and Development (R&D)
Research and Development (R&D) teams manage design controls, risk management, and validation documentation in a secure QMS that supports innovation without compromising compliance.
Key benefits are listed below.

Manufacturing and Operations
Manufacturing and operations teams manage batch records, equipment, suppliers, and deviations to ensure consistent, GxP-compliant production processes.
Key benefits are listed below.

Management and Executives
Management teams and executives gain a consolidated view of quality performance across departments to support informed decisions and continuous improvement.
Key benefits are listed below.

Scalable QMS Software for Startups, Growing Companies, and Large Enterprises
SimplerQMS provides a scalable, validated quality management software tailored to the needs of small businesses and startups, growing companies, and large life science enterprises.
SimplerQMS Case Studies and Reviews
See how life science teams of every size use SimplerQMS to simplify compliance and stay audit-ready, supported by real case studies and verified third-party reviews.
‘’SimplerQMS has been an excellent solution for our team. The platform is intuitive and user-friendly, and the implementation process was incredibly smooth. We were able to go live in just 6 weeks…’’
“The ease of use, customizability, and the support given by the technical support team…Very fast in their response.”
‘’SimplerQMS is much better than our previous paper-based QMS system…SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS and is easy to use.’’
“…I have consistently been impressed with its flexibility and range of features. These features have met all my needs in every aspect. The excellent support team further enhances the overall experience.”
“Amazing customer support and help. The system is clearly structured. After the introduction, it is easy to use.”
‘’User-friendly and simple to use. Easy to customize to fit your individual needs.’’
‘’Exceptional customer support! Compliant to 21 CFR Part 11 and ISO 13485:2015.’’
‘’…SimplerQMS offers the bare necessities and is perfect for our needs.’’
Why Companies Choose SimplerQMS Over Other QMS Software Alternatives?
Listed below are the reasons why companies choose SimplerQMS over other QMS software alternatives.
Meet the Life Science Experts Behind SimplerQMS
Founded by former leaders from Ambu (Jacob Sjørslev Hyrdum and Allan Murphy Bruun), SimplerQMS understands the real challenges of the life science industry.
We are more than a fully validated QMS software provider – we provide expert guidance, fast implementation, and practical, compliance-focused support.
Our team is committed to helping life science organizations achieve compliance, efficiency, and confidence in their quality systems.
Learn more about SimplerQMS
Get a Personalized Demo of SimplerQMS
A personalized demo just for you – we spend 3+ hours preparing to make it relevant.







Talk to Quality Solution Experts
30+ years of QA/RA experience
How to Choose the Best QMS Software for Life Science Companies?
Follow these steps to choose the right QMS software for your life science company.
Define Regulatory Requirements
The first step in selecting the right QMS software is to clearly define your organization’s regulatory, quality, and business requirements.
Identify all applicable regulations, standards, and guidelines relevant to your industry and ensure the QMS software is designed to align with these requirements. Depending on your life science segment, this may include general guidelines such as ISPE GAMP 5 and ISO 9001:2015, as well as industry-specific standards like ISO 13485:2016 (medical devices), ICH Q10 (pharmaceuticals), or ISO 15189:2022 (medical laboratories).
Evaluate Industry Fit
Choose a QMS solution specifically designed for your specific life science segment, whether pharmaceuticals, medical devices, biotech, or medical laboratories.
Industry-focused QMS software better reflects regulatory expectations, quality workflows, and terminology, reducing configuration and validation effort and implementation risk compared to generic QMS platforms.
Validate the System
Software validation is critical for regulated industries. Verify that the vendor provides a fully validated system under GAMP 5 guidelines with IQ/OQ/PQ documentation included.
A vendor-maintained validated system significantly reduces internal validation effort, resource consumption, and ongoing compliance burden.
Compare Core Features and Integration Capabilities
Once requirements are defined, gather detailed information from QMS vendors to support informed decision-making. Ensure the system includes the quality management module needed and integrates seamlessly with other QMS modules, Microsoft 365, ERP, LIMS, CRM, and relevant platforms.
This is commonly done through the following.
- Request for Information (RFI): Collects high-level information about features, services, and pricing models.
- Request for Quotation (RFQ): Requests specific pricing based on users, scope, and modules.
- Request for Proposal (RFP): Requires vendors to demonstrate how they will meet defined requirements, including implementation, support, and timelines.
Additionally, ensure the QMS software includes the core quality modules you need, such as document control, change management, training, CAPA, audits, and risk management. Using structured comparison tools, such as a QMS software comparison template, can help evaluate solutions side by side.
Evaluate Total Cost and Scalability
Review the vendor’s pricing model for transparency and scalability. Prefer an all-inclusive subscription that covers services such as hosting, validation, support, training, and future upgrades to avoid hidden costs.
During evaluation, assess the following aspects.
- Total cost of ownership (TCO)
- Scalability for future growth
- Support availability and response times
- Vendor roadmap and long-term viability
Review Customer Success and Support
Evaluate vendor credibility by reviewing customer feedback on platforms such as G2, Capterra, GetApp, and similar sources. Customer reviews provide real-world insights into usability, reliability, support quality, audit readiness, and overall value.
Furthermore, choose a partner with proven life science expertise, 24/7 global support, and a strong track record of successful audits and implementations.
Request a Personalized Demo
Schedule a demo to evaluate ease of use, configuration flexibility, and alignment with your quality and regulatory workflows. A tailored demonstration shows how well the system aligns with your compliance and operational goals.
Negotiate Terms and Make the Final Decision
During final negotiations, address pricing, contract terms, implementation timelines, SLAs, training, customization needs, and compliance obligations such as data privacy requirements.
The final decision should better reflect the following.
- Alignment with regulatory and business requirements
- Stakeholder consensus across quality, IT, and management
- Confidence in long-term scalability and vendor partnership
Discover Everything There Is to Know About QMS, Compliance, and More
Explore insights and best practices on QMS, compliance, and quality management for life sciences.
Frequently Asked Questions About Quality Management Software
Listed below are frequently asked questions about quality management software.
What Is Quality Management Software?
Quality Management Software (QMS software) is a digital platform that helps organizations manage, automate, and document quality management processes in line with relevant industry and regulatory requirements.
QMS software integrates document control, training, CAPA, change management, audits, risk management, and more into a single platform. QMS software helps life science companies support inspection readiness and align operations with GxP requirements through streamlined workflows and available modules.
QMS software is commonly used to digitize, manage, and support the policies, processes, and records defined within a Quality Management System (QMS). Some organizations continue to manage QMS records using paper-based QMS systems or separate point solutions due to regulatory scope or budget considerations.
Why Is QMS Software Important for Life Science Companies?
QMS software for life science companies is important because it enforces operational traceability, customer and regulatory compliance, ensures data integrity, and drives continuous process improvement.
Some benefits of QMS software are listed below.
- Regulatory Compliance: Maintains alignment and compliance with relevant requirements such as FDA, ISO, EU regulations, and more.
- Data Integrity and Traceability: Captures every action in secure, time-stamped audit trails, with electronic signatures, version control, and role-based access controls (RBAC) for full traceability.
- Efficiency and Automation: Replaces manual paperwork with automated workflows that speed up document reviews, training assignments, reviews, and approvals.
- Cross-Functional Collaboration: Connects teams across R&D, QA, manufacturing, and regulatory affairs through a single platform for shared visibility and faster decision-making, while supporting remote collaboration and global regulatory compliance.
- Continuous Improvement: Tracks nonconformances/deviations, CAPAs, and audit findings, and uses built-in analytics and quality dashboards to identify trends, reduce recurring issues, and drive process optimization.
- Cost and Resource Savings: Reduce overhead associated with processes such as manual validation, compliance reporting, and training matrix management. QMS software reduces labor costs, accelerates audits, and minimizes production downtime caused by documentation delays.
- Audit and Inspection Readiness: QMS software keeps all controlled documents, SOPs, and quality records centralized and searchable. Features like audit trail exporting, validation documentation, and automated reminders help ensure preparedness for internal audits, regulatory inspections, and sponsor audits.
What Types of QMS Solutions Are Used in Life Sciences?
The types of QMS solutions used in life sciences are described below.
- Paper-Based QMS: A manual system relying on physical documents.
- Spreadsheet or File-Based QMS: Uses tools like Excel, shared folders, and basic templates for quality documentation.
- On-Premise QMS Software: QMS software installed locally within the company’s IT infrastructure or company servers.
- Cloud-Based QMS Software: QMS software deployed in a secure, validated cloud environment, maintained by the vendor.
- Fully Validated SaaS QMS Software: Pre-configured and pre-validated under ISPE GAMP 5 guidelines, including all the quality management process modules.
How Does Life Science QMS Software Work?
A life science QMS software works by centralizing and streamlining all quality management processes within a validated digital system. QMS software connects quality management modules such as document control, training, change control, CAPA, audit management, and more, through integration of each process to one another for traceable electronic documents and records.
Furthermore, life science QMS software manages quality operations by automating document handling, tracking user actions, and limiting access to authorized, trained personnel, while remaining fully aligned with relevant regulatory and customer requirements.
What Core Quality Processes Does a Life Science QMS Software Support?
The core quality processes supported by a life science QMS software include the following.
- Document Control: Document control in a QMS platform enables the controlled process flow of documents, such as SOPs, policies, and protocols, through defined workflows for creation, approval, versioning, obsolescence, and periodic review. Document control helps maintain quality records and appropriately stores them to ensure data integrity.
- Change Management: Quality management software supports impact-assessed change management, capturing proposed changes to products, processes, or systems.
- Training Management: QMS system software allows for managing training requirements aligned with job functions through a user training matrix and role-based training assignments. Training management facilitates real-time tracking of completion status, helps ensure training effectiveness verification, and supports audit readiness by maintaining complete training histories.
- Deviation and Nonconformance Management: Deviation and nonconformance management enables capture, logging, and investigation of events where products, processes, or systems deviate from approved procedures or specifications.
- Complaint Management: Complaint management allows to manages product complaints by documenting, investigating, and resolving issues related to service issues, product performance, or safety.
- Corrective Action and Preventive Action (CAPA) Management: The CAPA management in a QMS software enables the identification, resolution, and prevention of issues by implementing corrective and preventive actions.
- Equipment Management: Equipment management helps maintain calibration, qualification, and maintenance of equipment to ensure accurate and consistent operation.
- Production and Process Control: Production and process control help ensure manufacturing processes are performed consistently according to documented procedures, with appropriate controls and monitoring to ensure that output meets specified requirements.
- Supplier Management: Supplier management allows for the qualification, monitoring, and auditing of suppliers to ensure material and service compliance with requirements.
- Risk Management: Risk management enables the identification and mitigation of risks across product lifecycle stages using structured risk assessment tools.
- Audit and Inspection Management: Audit and inspection management supports planning, execution, and documentation of internal audits, while tracking findings, corrective actions, and follow-up activities.
- Management Review: Quality management software streamlines management review processes by providing dashboards, KPI tracking, and trend analytics. This enables executives to make data-driven strategic decisions about the effectiveness of the quality management system.
What Third-Party Integrations Are Supported by Life Science QMS Solutions?
The various third-party integrations that life science QMS solutions support are the following.
- Microsoft 365: Enables real-time editing, collaborative reviews, and 21 CFR Part 11–compliant approvals directly within Word, Excel, and PowerPoint. Includes version control, RBAC, and audit trail integration for secure document workflows
- Enterprise Resource Planning (ERP) Systems: Integrates supplier qualification, material inventory, and production lot data with the QMS. Supports bi-directional data flow to align manufacturing and quality operations, enhancing GxP compliance and batch traceability.
- Customer Relationship Management (CRM) Systems: Connects customer complaints, nonconformance reports, and feedback tracking to the QMS. Ensures timely resolution and complete CAPA documentation, with traceable communication logs for audit support.
- Laboratory Information Management Systems (LIMS): Links test results, COAs, and lab protocol data with quality records. Enables data integrity and audit readiness, with automated record syncing for lot release, stability, and environmental monitoring programs.
- Electronic Lab Notebooks (ELN): Supports seamless experiment documentation, version control, and e-signature capture from early-stage research. ELN integration ensures that research-to-development workflows remain traceable and compliant.
- Product Lifecycle Management (PLM) Systems: Connects design history, engineering change orders, and regulatory submission artifacts with QMS processes. Particularly valuable for biotech and medical device companies managing ISO 13485 and FDA Design Control requirements.
- Manufacturing Execution Systems (MES): Enables real-time synchronization of batch records, process parameters, and production deviations into QMS workflows. Supports FDA 21 CFR Part 11, EU GMP Annex 11, and other applicable requirements through validated data exchange.
- eSignature and Authentication Tools: Ensures 21 CFR Part 11–compliant, user login and authentication, with timestamped audit trails and SSO compatibility. Provides secure approvals and digital identity assurance for global, cross-functional teams.
- API Integrations: Supports flexible APIs and webhooks for scalable interoperability with clinical, regulatory, IT, and R&D platforms. Validated integration ensures data harmonization, compliance with controlled vocabulary, and alignment with validation lifecycle documentation (IQ/OQ/PQ).
How Much Does QMS Software Cost?
The cost of QMS software for life science companies varies based on deployment model, compliance requirements, user volume, and the level of validation documentation included. Here are the most common pricing models and what they typically include are described below.
- Subscription-Based (SaaS) Model: Companies pay a monthly or annual subscription fee that includes hosting, 21 CFR Part 11-compliant e-signatures, IQ/OQ/PQ validation packages, support, and automatic software upgrades. Ideal for small to mid-sized companies seeking fast deployment, low IT burden, and predictable total cost of ownership (TCO).
- Per-User or Tiered Licensing Model: Pricing is based on the number of active users or feature access tiers. This model supports scalability, allowing teams to grow over time. Some vendors offer RBAC and license management tools to align user permissions with cost optimization.
- On-Premise License Model: Involves a one-time software license fee, with ongoing support and maintenance costs. The on-premise license model requires internal IT infrastructure for server hosting, GAMP 5 validation, and manual system updates. Preferred by larger enterprises or CDMOs with existing tech teams and multi-site deployment needs.
- Modular Pricing Model: Offers à la carte access or pay for specific QMS modules to individual QMS components such as CAPA management, document control, or training matrix automation. Cost-effective for startups or CROs with focused needs, but can lead to higher costs as more modules are added during expansion.
- All-Inclusive Pricing Model: A flat-rate subscription that includes all QMS modules, validation documentation, SOP automation, audit trail tracking, and customer service support. This pricing model offers maximum transparency and reduces the risk of hidden upgrade fees or feature gaps. Ideal for regulated life science companies pursuing end-to-end GxP compliance.
SimplerQMS offers subscription-based, transparent pricing that covers everything life science companies need to achieve compliance and operational efficiency, including full implementation and validation, as well as hosting, training, and support. No hidden fees, no add-ons, and full access to every QMS module.
The license types of SimplerQMS are listed below.
- Viewer License: The Viewer license is free of charge with typical users, such as auditors, consultants, or external collaborators, for easy, read-only access to the documents they need.
- Light License: The Light license is for users who need to initiate quality events, sign documents, search for information, and complete training. Light license is usually recommended to production operators, laboratory technicians, or executives who require limited functionality at a lower cost.
- Standard License: The Standard license includes document control plus any two QMS modules of your choice. Perfect for production managers, engineers, or document control specialists working in specific quality areas.
- Full License: The Full license provides access to all QMS modules and administrative features. Ideal for QA, RA, and site heads who manage compliance across the organization.
What Does SimplerQMS Pricing Include?
SimplerQMS pricing includes everything required to implement and operate a fully validated quality management software, including implementation, validation, user training, cloud hosting, and continuous expert support. Flexible license options ensure each team member has the appropriate level of access while keeping your setup efficient and cost-effective.
This transparent QMS software pricing model supports predictable budgeting, faster Return on Investment (ROI), and a ready-to-use system that scales with your organization’s growth.
What Are the Prerequisites for Implementing a QMS Platform?
The prerequisites for implementing a QMS platform are discussed below.
- Defined Quality Objectives: Establish measurable quality goals that align with business strategy, regulatory requirements, and process optimization targets.
- Regulatory Understanding: Identify and interpret applicable requirements, including FDA 21 CFR Parts 11, 210, 211, 820, EU MDR/IVDR, ISO 13485, and GxP guidelines. Regulatory understanding forms your regulatory intelligence foundation, ensuring the QMS implementation meets global compliance expectations.
- Process Documentation: Map and assess existing quality workflows such as CAPA, change control, document management, and deviation/nonconformance handling. Process documentation supports effective system configuration and highlights areas for workflow automation and digital transformation.
- Data Readiness: Prepare legacy documents, SOPs, and records for migration. Data readiness includes performing data validation, defining a document migration protocol, and verifying version control for audit traceability.
- Cross-Functional Team Alignment: Assemble a core team from QA, RA, manufacturing, and IT to drive user adoption and ensure alignment across all system touchpoints. Include roles like system owner or system administrator, computer system validation (CSV) lead, and business process owners.
- Management Commitment: Secure executive support for budget allocation, resource planning, and cross-department engagement. Management must endorse the QMS as part of the organization’s quality culture and compliance strategy.
- Validation Strategy: Define your CSV approach in accordance with ISPE GAMP 5. Validation strategy includes an IQ/OQ/PQ plan, a user requirements specification (URS), and system configuration documentation to ensure sustained audit-readiness.
- Training Plan: Create a structured training matrix for all users, tailored by role, responsibility, and workflow access. Training should cover QMS functionality, regulatory expectations, and change management protocols, ensuring compliance with applicable requirements and a successful go-live.
How Do Life Science Companies Implement SimplerQMS Software?
Life science companies implement SimplerQMS through five key phases, typically completing implementation within 5–6 weeks.
- Kick off the project. Begin with a dedicated onboarding session led by SimplerQMS implementation specialists to define the scope, objectives, and success criteria for your organization.
- Configure and migrate data. Set up your validated environment using predefined templates and workflows while securely migrating existing quality documentation into SimplerQMS.
- Validate the system. SimplerQMS delivers a fully validated system that complies with GAMP 5 guidelines, complete with IQ, OQ, and PQ documentation, eliminating the need for internal validation work and resources.
- Train your team. Comprehensive user training ensures your team can manage documents, CAPAs, audits, and other quality management processes efficiently inside the SimplerQMS platform. Training includes live sessions, videos, and sandbox environments.
- Go live and receive ongoing support. Deploy the validated QMS system software and receive continuous support from SimplerQMS expert guidance, unlimited training, and ongoing support to maintain compliance, optimize adoption, and achieve lasting success.
How Long Does It Take to Implement a Cloud-Based QMS?
The timeline for a cloud-based QMS implementation can vary depending on several factors. In SimplerQMS, the timeline typically takes 6 weeks or more for life science companies. This timeline includes system setup, configuration, initial user onboarding, and full implementation.
What factors can affect the time taken for implementing a cloud-based QMS?
Several factors influence the total implementation timeline for a cloud-based QMS, including the following.
- Company Size and Structure: Larger organizations, especially those with multi-site operations, require more time for configuration, user role mapping, and data access controls. Cross-department coordination can extend project timelines without clear ownership and system governance.
- Process Complexity: Additional time will be needed for workflow configuration and URS documentation under GAMP 5 guidelines if your company has numerous quality workflows (e.g., CAPA, change control, deviation, audit management) or custom SOP lifecycles.
- Data Migration Scope: The quantity and condition of legacy quality records, SOPs, and training data directly affect migration time. Steps such as data cleansing, file mapping, and document version verification take time and must be planned during the pre-implementation phase.
- Regulatory Requirements: Compliance with applicable and relevant requirements adds complexity. Activities such as IQ/OQ/PQ validation, audit trail configuration, and eSignature readiness often extend project timelines unless using a pre-validated, compliant platform tailored to your organization’s industry.
- User Training and Adoption: Companies implementing QMS across multiple departments or regions may require planned and phased onboarding. Developing a training matrix, assigning role-based workflows, and ensuring change management acceptance are all essential time drivers for implementation to go live.
- Internal Resource Availability: Delays often stem from limited involvement by QA, RA, IT, or process owners. To maintain momentum, a cross-functional project team must be allocated with clear responsibilities and timelines for configuration planning and reviews.
- Vendor Support and Validation Readiness: Choosing a QMS software vendor with a GAMP 5–validated SaaS QMS and pre-packaged validation documentation significantly reduces setup time. Features such as prebuilt templates, audit-ready, configured files, and 24/7 customer support accelerate go-live readiness.
See SimplerQMS in Action
To see SimplerQMS in action and learn how you can make the most of it, request a personalized demo presentation.
Book a Demo







