QMS Software for Contract Research / Manufacturing Organizations (CRO / CMO)
Streamline operations and maintain regulatory compliance with purpose-built quality management software for Contract Research / Manufacturing Organizations (CRO / CMO).
TRUSTED BY
























Ensure Regulatory Compliance in Your Contract Organization
Contract manufacturers, clinical research organizations, and testing laboratories (CxOs) must comply with the same regulatory requirements as their clients in the pharmaceutical, biotechnology, medical device, and other life science industries.
CxOs must maintain compliance with relevant standards, regulations, and regulatory guidelines in Life Sciences depending on the clients they serve such as GxP, FDA 21 CFR Part 11, 210, 211, and 820, ISO 13485:2016, ICH Q10, EU MDR and IVDR, EU GMP Annex 11, ISO 9001:2015, and others.
Compliance with these requirements can be achieved easier and faster with a purposefully built QMS software solution for Contract Organizations from SimplerQMS.
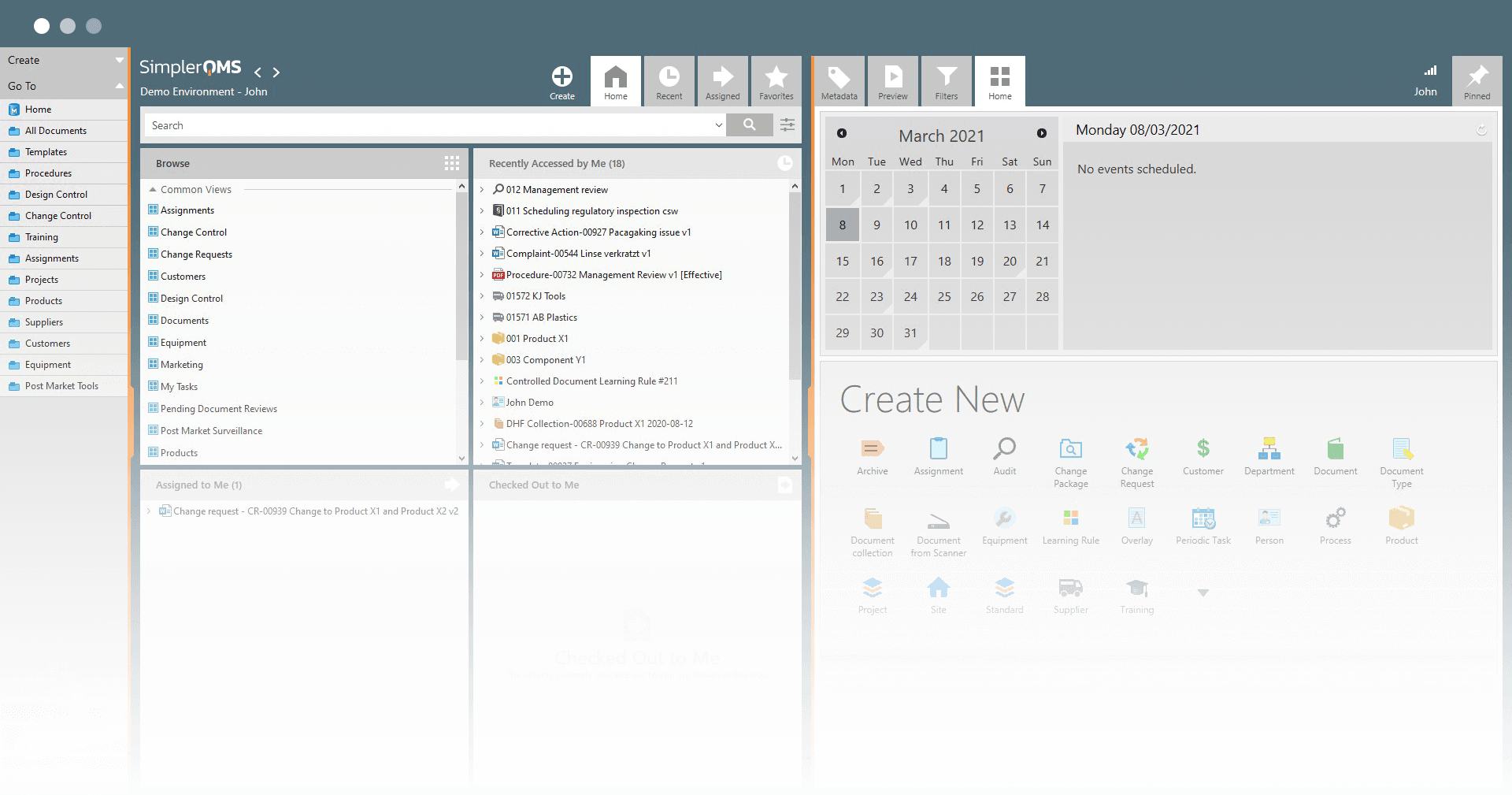
One Platform That Integrates All Quality Processes
With the SimplerQMS software solution, you can streamline regulatory compliance and typical CRO / CMO quality control processes by automating repetitive documentation activities.
Integrate all your quality assurance and control processes, store data in centralized, cloud storage, and manage risks, training, audits, suppliers, and other processes with ease.
Work More Efficiently in Microsoft Office Environment
SimplerQMS integrates with native Microsoft Office applications, including Word, Excel, Outlook, and PowerPoint, so you and your team can work with the tools you’re already familiar with.
Simply work on your documents within Microsoft Office applications and once the work is done, save them with one click in SimplerQMS Cloud. No manual download and upload of documents are required.
Streamline Your Document Control Processes
Automate document routing, follow-ups, tracking, escalation, review, approval, and other document-based processes.
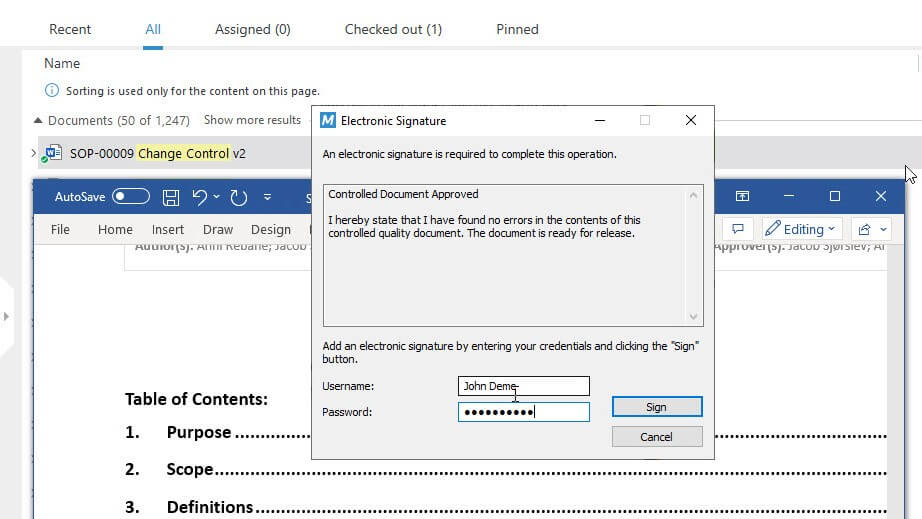
With features like time-stamped audit trails and FDA 21 CFR Part 11 compliant electronic signatures built into the SimplerQMS solution, you can be confident that all documents are processed efficiently and follow the applicable regulatory requirements.
Forget About Time-Consuming Validation Activities
Software validation is a requirement for CROs and CMOs as well as other life science organizations that store and manage quality records electronically.
SimplerQMS provides a fully validated solution and performs monthly re-validation of the system, in compliance with FDA 21 CFR Part 11, ISO 13485:2016, and GxP guidelines. This means that your organization won’t have to spend time on system validation processes.
What Validation Documentation Evidence Does SimplerQMS Provide?
- Validation Procedure
- Validation Plan
- Validation Report
- IQ, OQ and PQ Documentation
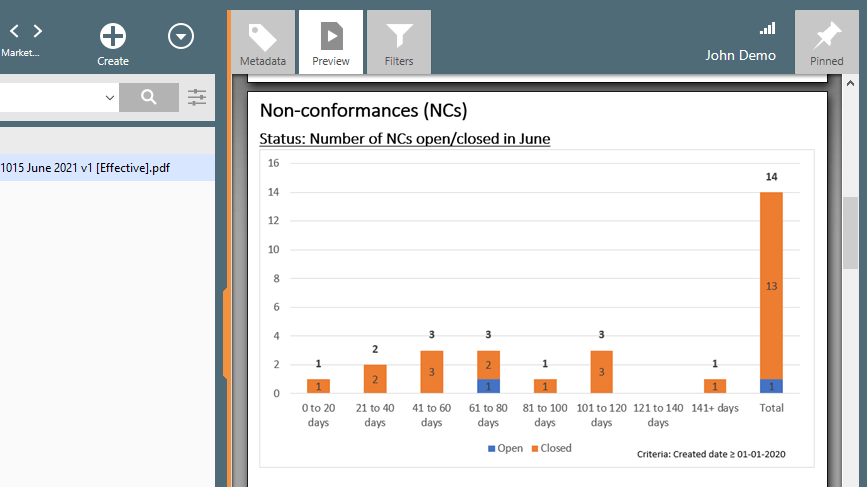
Manage Nonconformance More Easily
The SimplerQMS nonconformance management solution facilitates the process of identifying, evaluating, analyzing, and managing non-conformances.
Access a single source of truth where all processes are connected from non-conformances and other quality events to training, audit findings, and more.
Resolve Customer Complaints Quickly and Effectively
As a CRO / CMO, it’s of the highest importance for you to meet the requirements of your main customers without delays and quickly resolve any complaints.
SimplerQMS provides robust customer complaint-handling capabilities which allow you to track, manage, analyze and resolve customer complaints in a timely and effective manner.
Automate and Gain Traceability of Your CAPA Processes
The SimplerQMS CAPA management software solution integrates with different QMS sub-systems and provides a closed-loop CAPA process.
Save time with automated CAPA workflows and pre-configured CAPA forms, and avoid any errors in manual data entry or process execution.
Manage Changes More Effectively
The change control module by SimplerQMS allows you to automatically track changes, collect data, and send changes through an approval process.
Automated follow-ups, notifications, and escalation activities help you quicker finalize changes to SOPs in your system.
Expedite Employee Training Management Processes
With continuous changes taking place daily, you must ensure that employee training in your contract organization is well-documented and in compliance with regulatory requirements.
SimplerQMS training management module streamlines training processes by automating the assignment of training activities, reminders, generation of training certificates, and more.
All-in-One QMS Software for Life Sciences
Document Control
Manage documents in a centralized system with full control, visibility, and traceability of document versioning, approvals, and distribution.
Employee Training
Efficiently manage employee training records, automate training assignments and tasks, and ensure compliance with quality standards.
Change Control
Automate and manage change requests, approvals, and implementation processes to avoid disruptions in your quality system.
Non-Conformances
Track, manage, and resolve non-conformances in a timely and effective manner before they turn into CAPAs.
CAPA Management
Identify, investigate, correct and prevent quality issues with our CAPA management software solution.
Audit Management
Efficiently plan conduct, and track audits, as well as manage audit findings with an all-in-one QMS software.
Frequently Asked Questions
Why Is Quality Management Important for CROs and CMOs?
As a Contract Research / Manufacturing Organization (CRO / CMO), you are responsible for ensuring that the services you provide meet customer expectations and regulatory requirements. An effective quality management system (QMS) is essential to help you manage your processes and ensure compliance. By implementing a QMS, you can streamline your operations, improve communication and collaboration between different departments, and ultimately provide better quality services to your customers.
What Are the Benefits of QMS Software for CROs and CMOs?
QMS software solutions offer many benefits for Contract Organizations, including streamlined operations and improved efficiencies, reduced paper documentation and associated costs, increased visibility into quality data for better decision making, improved compliance with regulatory requirements, and enhanced communication between sites, sponsors, and staff.
Is SimplerQMS Solution Software Validated?
Yes, SimplerQMS provides a fully validated software solution. It is validated according to ISPE GAMP5 and undergoes revalidation for each new version or standard update.
We do all the software validation for you – there are no additional expenses, resources, or time commitments to software validation on your part.
SimplerQMS complies with requirements regarding the validation of systems used for QMS such as 21 CFR Part 11, and 820, EU GMP Annex 11, and ISO 13485:2016.
Furthermore, we provide extensive validation evidence for use during audits and inspections, including the validation plan, procedures, and reports, as well as IQ, OQ, and PQ documentation.
How Much Do SimplerQMS QMS Tools Cost?
SimplerQMS provides an all-in-one QMS software solution that includes all modules, implementation, training, ongoing support, validation, hosting, and more. With SimplerQMS, you pay one price that covers everything – there are no other subscription fees.
The exact price of the solution differs depending on how many people will use it, and the license types required. Therefore we recommend you book a tailored demo and talk to our experts to request a pricing quote for the needs of your organization.
How Much Time Does It Take to Implement SimplerQMS Software?
Depending on whether you already have a quality management system (QMS), and the number of documents, implementation time may be shorter or longer. Our experience shows that the average implementation time for a new QMS is between five and six weeks. However, we have seen clients go live in as little as three weeks.
Our team of experts will work with you every step of the way to ensure a smooth and successful transition to our software.
What Customers Achieve With SimplerQMS
Utilize Proven Technology
SimplerQMS is built on Microsoft & M-Files Technology which serves over 5,000 customers worldwide.
Pass audit more easily
Access needed documentation and present it to the auditor with a couple of clicks from anywhere in the world.
Gain high level of traceability
Gain cross-functional visibility and trace back to the root cause of each nonconformance.
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
See What Our Customers Have to Say
“Spending most of my day using SimplerQMS, I would say I am very pleased with the ease of use.”
Dorthe W.
QA/RA Manager, Cortex Technology
“SimplerQMS gave us excellent pricing, customer support for understanding how to use their system and set up our QMS, and is easy to use.”
Subba S.
Chief Technology Officer, CollaMedix
“Easy to work with. Intuitive. Rather easy to setup. Very good customer support. Good quality to price ratio.”
Jean Claude M.
Head of Hardware and Software Development, hemotune
See SimplerQMS in Action
To learn how you can make the most of SimplerQMS in your organization book a personalized demo.