Regulatory Information Management Software (RIMS) for Life Sciences
Centralize and manage regulatory information and submissions to improve visibility, traceability, and oversight.
Book a DemoJoin Over 5,000+ Quality Professionals Who Trust SimplerQMS
Generate Compliant, Error-Free Regulatory Submission Documentation.

Manage All Your Regulatory Information in a Single Platform
Nowadays, Regulatory Affairs teams operating in life science industries, such as medical devices, pharmaceuticals, biologics, and others, are tasked with managing an ever-growing amount of data and documentation. This can be daunting, especially when using multiple software systems to store and manage this data.
SimplerQMS Regulatory Information Management System (RIMS) provides a cloud-based solution for managing all your regulatory information in a single platform.
Role-based access control ensures that only authorized users have access to the data they need.
Seamlessly Collect Your Product Information Into Technical Dossiers
Collect and organize all the needed product information you need to submit product submissions for each global agency in their regulated formats.
Add submission-specific metadata to each file, so you can always find the right one quickly and easily. Relate product-related documents to multiple archives, for various regulatory submissions and avoid duplication of the same file.
When it’s time to compile your technical dossiers, such as electronic Common Technical Document (eCTD) dossiers – make a snapshot of current documentation for each product and share it externally.

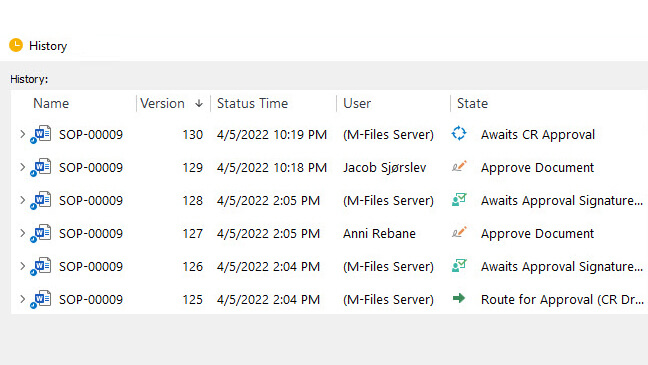
Automate and Streamline Regulatory Processes
Utilize controlled document workflows for document creation, routing, review, approvals, and escalation.
SimpleQMS fulfills regulatory requirements set forth by FDA 21 Part 11 and provides a complete, time-stamped audit trail for all document changes, version control, and electronic signatures.
Monitor and track the progress of individual tasks and activities with our visual workflow status feature. Take advantage of automatic email notifications and reminders to keep everyone informed, and never miss a regulatory deadline again.
Connect Regulatory Information Management (RIM) With Your QMS
SimplerQMS allows you to integrate the Regulatory Information Management System (RIMS) solution with your Quality Management System for a single source of truth.
For example, relate product registration records to the quality control procedures used for that product, or link clinical trial data to the marketing authorization application.
By integrating your Regulatory and Quality processes, you can avoid duplication of effort, save time, and manage all your quality and compliance-related activities in one place!
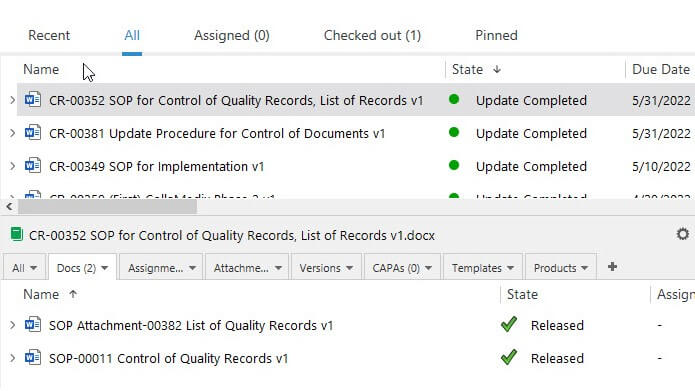
eQMS That Streamlines Quality & Regulatory Compliance
Frequently Asked Questions
A Regulatory Information Management System (RIMS) is a software solution that helps life science organizations manage their regulatory product information more efficiently and effectively.
The software enables organizations to collect, track and manage product dossiers and helps prepare for regulatory submissions, approvals, and product registrations in the required formats set forth by global regulatory agencies.
Some of the core features of an effective RIMS software include:
- Automated document workflows
- FDA 21 CFR Part 11 electronic signatures
- Time-stamped audit trails
- Version control and document change tracking
- User access controls and permissions
- Automated email notifications, task reminders, etc.
- Ability to collect & organize regulatory product information
Yes! SimplerQMS provides a pre-validated software solution that complies with the requirements of FDA 21 CFR Part 11, GxP, and ISO 13485:2016. This allows our customers to have peace of mind knowing that our software is compliant with industry standards.
We ensure continuous re-validation of the software, every month, and provide extensive validation evidence. This includes our validation plan, procedure, reports, IQ, OQ, and PQ documentation.
Yes, SimplerQMS RIMS software is 21 CFR Part 11 compliant, as it is designed to help organizations operating within life sciences comply with the Electronic Signature and Digital Record practices set forth by the FDA 21 CFR Part 11.
SimplerQMS RIMS solution is part of an all-in-one QMS software.
This includes support for all the core QMS system modules, system implementation, online training, ongoing support, continuous re-validation, cloud hosting, and more. This means that everything is included in the price you pay and there are no other costs associated with subscribing to SimplerQMS.
To learn more about our pricing, please contact us for a demo and price quote.
What Customers Achieve With SimplerQMS
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
