Change Control Management Software for Life Sciences
Streamline change control processes from request submission through review, approval, and implementation.
Book a DemoJoin Over 5,000+ Quality Professionals Who Trust SimplerQMS
Change Control Management Within a Complete eQMS
The change control management solution by SimplerQMS allows Life Science companies to create, document, and manage all changes within the organization.
To document the change process, you can create and delegate specific assignments that will be electronically signed when approved. Various features will be available to you, such as best-practice change request templates, the ability to specify documents in scope for the change, select workflow participants, routing changes for review and approval, setting reminders, and more.
SimplerQMS change control management software is part of a complete quality management software solution, which includes core life science QMS modules such as training, non-conformance, CAPA, supplier, audit management, and much more.
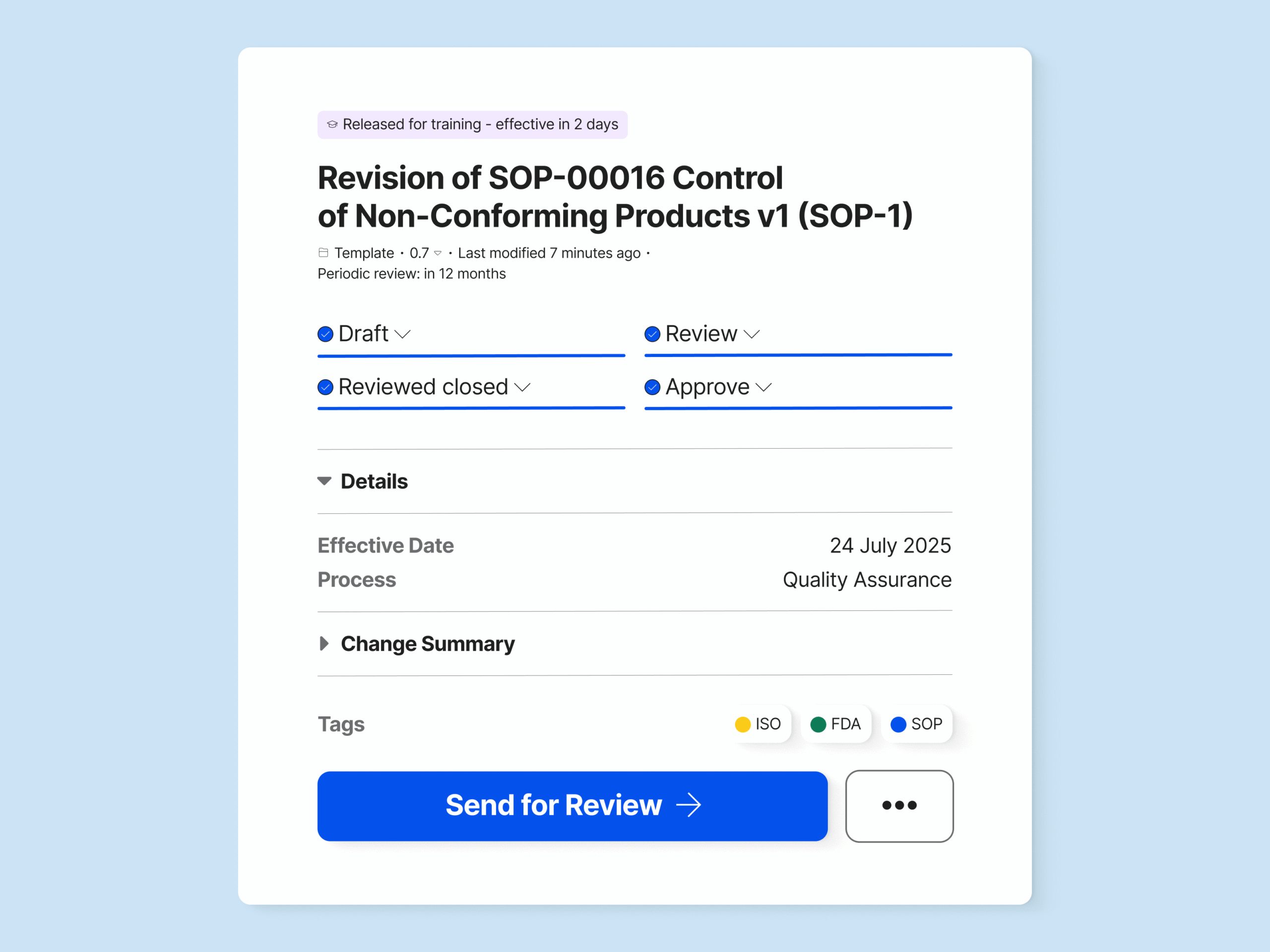
Create, Review, and Approve Change Requests
Use built-in templates to create change requests directly from, for example, non-conformances and CAPAs, saving time and simplifying the workflow. Or design your own change request templates to meet your specific needs.
Specify which document type needs to be updated with a change request, for example, an SOP, and which document types can be updated without a change request.
Easily route change requests for review or update and assign specific people to the task. Set up reminders to ensure the change request is conducted on time.
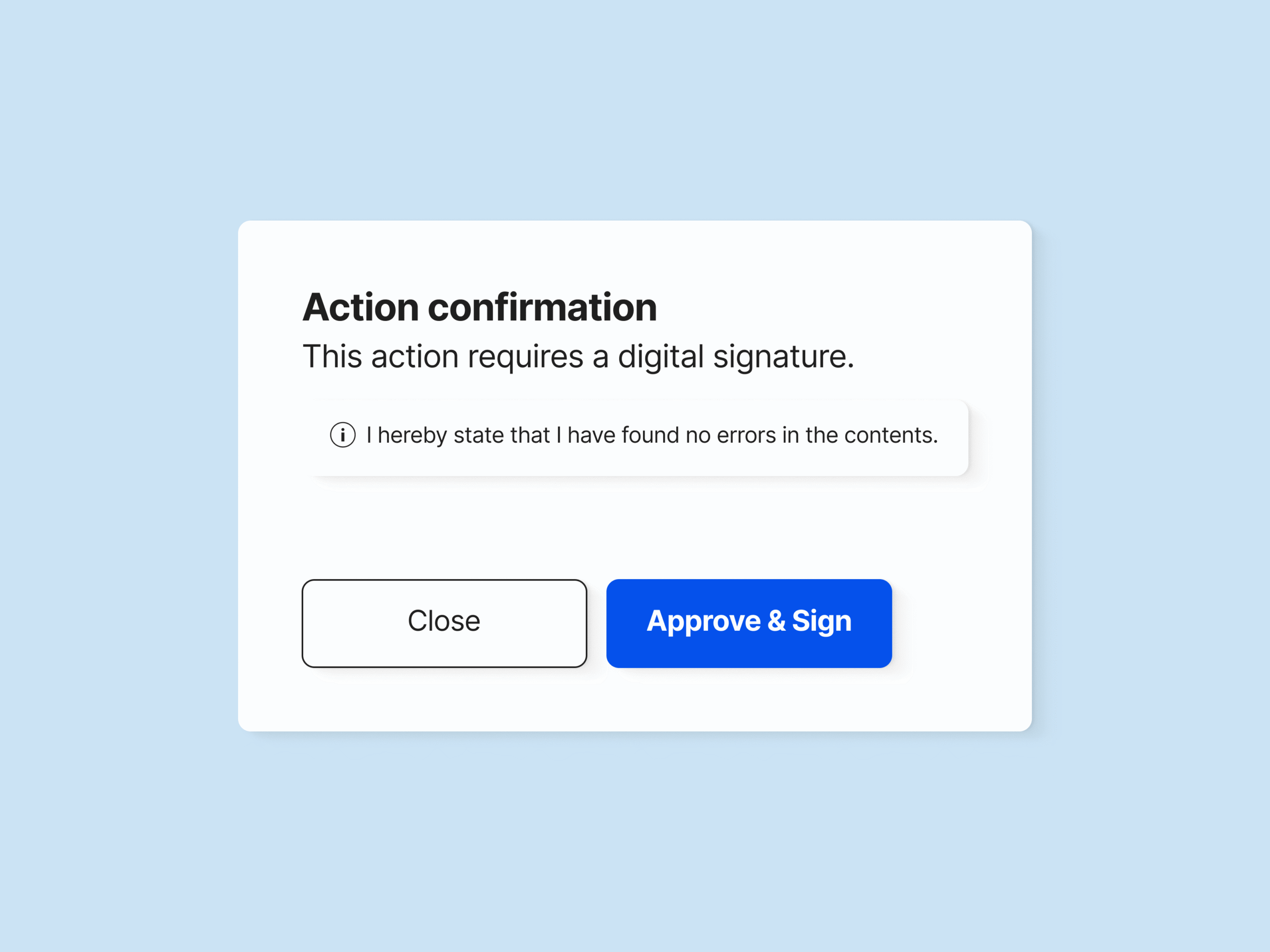
Electronically sign the change request upon approval to document the change justification and record all process steps.
Be Accountable for All Changes
SimplerQMS solution allows you to overview change request status via customizable views and monitor the progress of each change. The system automatically records all changes made to documents and provides complete time-stamped audit trails.
Store all data in a centralized, cloud-based solution for secure and easy access from anywhere. Retrieve data quickly and share with relevant collaborators with a few clicks.
Prevent unauthorized changes in documentation by setting access level security. Change requests for specific documents, for instance, Quality Manuals, can only be accessed by the Change Manager and assigned people.
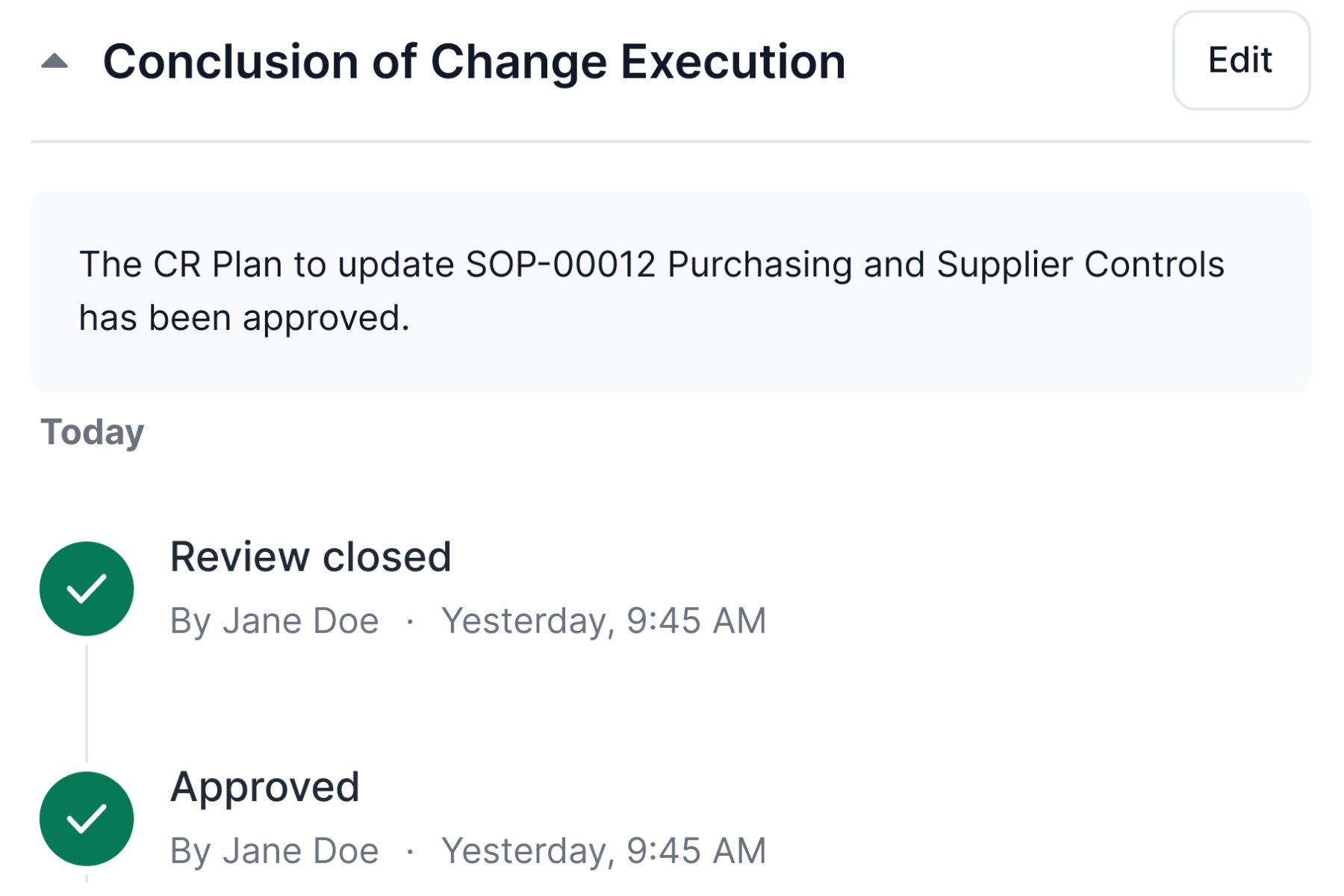
Ensure Compliance With Life Science Requirements
Monitor, control, and record changes efficiently across processes and documents and comply with Life Science requirements.
Change control management software simplifies change request workflow and supports companies’ continuous improvement processes.
SimplerQMS enables you to sign all documents and files using electronic signatures compliant with FDA 21 CFR Part 11 and EU GMP Annex 11.
Implement Change Across All Departments
Choose the documents in scope from different departments to create a seamless workflow. For example, Change Managers can generate a change request directly from a customer complaint to modify steps in an SOP later on.
Set up notifications and reminders allowing employees to keep track of change requests and their required activities, to help ensure that all change-related processes are completed on time.
Native integrations to other QMS modules such as CAPAs, non-conformances, document control, employee training, suppliers, audits, and many others enable all changes to be properly controlled and managed throughout the organization.


Raise Awareness and Keep Employees Updated
Raise awareness of employees regarding changes and updated documents in a timely manner with email notifications.
For example, you can also integrate change control management with employee training management to keep the workforce always trained and competent to perform their functions. Automatically notify relevant employees regarding new training activities after updating a document related to a change request.
Monitor Change Impact and Effectiveness
Change priority can be set in the metadata card according to your needs. For example, a change with an extensive impact on an SOP could be addressed first, with a high priority.
Automatically set assignments and tasks to streamline the process of assessing effectiveness, if needed.
Communicate changes and their impact on departments to help employees understand and implement change efficiently.

See Our Change Control Management in Action
SimplerQMS supports compliant, risk-based change control with customizable workflows tailored for your organization. See how to create change requests, link affected documents, and assign actions with automated notifications.
What Customers Achieve With SimplerQMS
“It’s very flexible, smooth, and easy to use. Documents no longer get lost and the whole history of all products is accessible for anyone at any time.”
Discover How SimplerQMS Can Help You
Beyond Just Change Control Management
Frequently Asked Questions
Change control management systems are designed to help companies streamline the process of controlling and managing changes to documents, products, templates, and processes. It allows companies to track changes, collect data and manage the approval process for any type of change in a secure and traceable manner.
Change control refers to the process of evaluating a change request and deciding if it should be approved. Change control is a part of change management for some Life Science companies.
Change management oversees the change implementation process to ensure changes are correctly implemented in the organization.
As the name suggests, change control management software provides a platform to track changes, collect data and send them through the approval process. With this software, companies can ensure that all changes are properly controlled and managed throughout the organization.
The change control management solution by SimplerQMS provides support for change management and control of changes.
You can create change requests and control changes to any documents, products, and templates. Specific whether document types such as an SOP, require a change request and which document types can be updated without it.
SimplerQMS allows you to use built-in or your change request templates, specify which documents are in scope for the change, select people who should participate in the workflow, route changes for review or approval, create and delegate assignments, set reminders, approve changes using electronic signatures, and more.
A change request in SimplerQMS starts with creating a change request document from one of our provided templates or your own personalized template. You may have change request templates for different purposes, for instance, one for document changes, another for product changes, and so on.
The software allows naming the changing request, linking documents and products that are in the scope of the change, and selecting any number of people to participate in the change request workflow.
Change requests can be edited in Microsoft Office Word or Excel and stored in the system cloud. When you check in a document, it automatically saves the changes and uploads the file into the system.
After the change request is drafted, you can route the document for review or approval. Participants will be automatically notified of required actions.
The system automatically keeps track of all document changes, which can be viewed through a complete time-stamped audit trail.
The change request approval is then electronically signed to document responsibilities and change justifications.
Yes, SimplerQMS is a software system compliant with FDA 21 CFR Part 11 and EU GMP Annex 11 regarding electronic signatures.
As part of change control and change management process requirements, all alterations made to documents and products must be signed to document responsibilities and change justification.
Usually, it takes 5 to 6 weeks to implement the SimplerQMS solution.
Implementation times can vary depending on the number of documents created or migrated within the software and available resources.
The SimplerQMS change control management software is part of a complete eQMS solution. You can take advantage of all the core life science QMS modules, including equipment management, CAPA, training, audits, risk management, and others.
We offer a complete solution that includes all QMS modules, implementation, user training, hosting, ongoing support, and more. The total cost will vary based on how many licenses you acquire.
Visit our pricing page to learn more about what is included.
